The Perceval Sutureless Bioprosthetic Aortic Valve: Evolution of Surgical Valve Technology
- PMID: 38465600
- PMCID: PMC11055413
- DOI: 10.1177/15569845241231989
The Perceval Sutureless Bioprosthetic Aortic Valve: Evolution of Surgical Valve Technology
Abstract
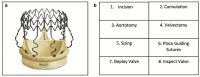
Objective: The surgical treatment of aortic stenosis continues to evolve, and sutureless aortic valve replacement (SUAVR) is an emerging technology. With the Perceval S (Corcym, London, UK) as the only true sutureless valve on the market, the objective of this review is to analyze the current literature on Perceval S. Focusing on valve design and deployment as well as applications of the technology for challenging pathology, clinical outcomes are assessed, including a comparison with transcatheter AVR (TAVR).
Methods: PubMed and MEDLINE were searched by 3 authors for studies analyzing SUAVR from inception to May 19, 2023.
Results: SUAVR facilitates minimally invasive surgery and offers an alternative strategy for patients with small aortic annuli. It also has a time-saving advantage for patients who require complex operations. SUAVR results in excellent long-term morbidity, mortality, durability, and hemodynamic function. In comparison with conventional surgical AVR (SAVR), SUAVR does have a greater risk of postoperative pacemaker implantation; however, increasing user experience and refinements in implantation technique have contributed to reductions in this outcome. SUAVR results in morbidity and mortality that is similar to rapid-deployment AVR. Midterm outcomes are superior to TAVR; however, further robust investigation into all of these comparisons is ultimately necessary.
Conclusions: SUAVR bridges the gap in technology between SAVR and TAVR. The application of this exciting technology will undoubtedly grow in the coming years, during which additional investigation is paramount to optimize preoperative planning, valve deployment, and reintervention strategies.
Keywords: minimally invasive aortic valve replacement; rapid deployment aortic valve replacement; sutureless aortic valve replacement.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Roth GA Mensah GA and Fuster V.. The global burden of cardiovascular diseases and risks: a compass for global action. J Am Coll Cardiol 2020; 76: 2980–2981. - PubMed
-
- Eveborn GW, Schirmer H, Heggelund G, et al.. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart 2013; 99: 396–400. - PubMed
-
- Otto CM, Nishimura RA, Bonow RO, et al.. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143: E72–E227. - PubMed
-
- Smith CR, Leon MB, Mack MJ, et al.. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187–2198. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous