A binding site for phosphoinositides described by multiscale simulations explains their modulation of voltage-gated sodium channels
- PMID: 38465747
- PMCID: PMC10928511
- DOI: 10.7554/eLife.91218
A binding site for phosphoinositides described by multiscale simulations explains their modulation of voltage-gated sodium channels
Abstract
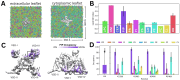
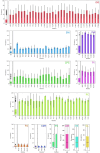
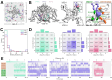
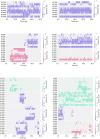
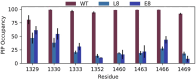
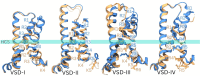
Voltage-gated sodium channels (Naᵥ) are membrane proteins which open to facilitate the inward flux of sodium ions into excitable cells. In response to stimuli, Naᵥ channels transition from the resting, closed state to an open, conductive state, before rapidly inactivating. Dysregulation of this functional cycle due to mutations causes diseases including epilepsy, pain conditions, and cardiac disorders, making Naᵥ channels a significant pharmacological target. Phosphoinositides are important lipid cofactors for ion channel function. The phosphoinositide PI(4,5)P2 decreases Naᵥ1.4 activity by increasing the difficulty of channel opening, accelerating fast inactivation and slowing recovery from fast inactivation. Using multiscale molecular dynamics simulations, we show that PI(4,5)P2 binds stably to inactivated Naᵥ at a conserved site within the DIV S4-S5 linker, which couples the voltage-sensing domain (VSD) to the pore. As the Naᵥ C-terminal domain is proposed to also bind here during recovery from inactivation, we hypothesize that PI(4,5)P2 prolongs inactivation by competitively binding to this site. In atomistic simulations, PI(4,5)P2 reduces the mobility of both the DIV S4-S5 linker and the DIII-IV linker, responsible for fast inactivation, slowing the conformational changes required for the channel to recover to the resting state. We further show that in a resting state Naᵥ model, phosphoinositides bind to VSD gating charges, which may anchor them and impede VSD activation. Our results provide a mechanism by which phosphoinositides alter the voltage dependence of activation and the rate of recovery from inactivation, an important step for the development of novel therapies to treat Naᵥ-related diseases.
Keywords: inactivation; ion channel; lipid-protein interaction; molecular biophysics; molecular dynamics; none; phosphoinositide; sodium channel; structural biology.
© 2023, Lin, Tao et al.
Conflict of interest statement
YL, ET, JC, BC No competing interests declared
Figures










Update of
- doi: 10.1101/2023.07.16.547149
- doi: 10.7554/eLife.91218.1
- doi: 10.7554/eLife.91218.2
Similar articles
-
Regulation of Na+ channel inactivation by the DIII and DIV voltage-sensing domains.J Gen Physiol. 2017 Mar 6;149(3):389-403. doi: 10.1085/jgp.201611678. Epub 2017 Feb 23. J Gen Physiol. 2017. PMID: 28232510 Free PMC article.
-
Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels.J Gen Physiol. 2013 Aug;142(2):101-12. doi: 10.1085/jgp.201310998. Epub 2013 Jul 15. J Gen Physiol. 2013. PMID: 23858005 Free PMC article.
-
The S4-S5 linker couples voltage sensing and activation of pacemaker channels.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11277-82. doi: 10.1073/pnas.201250598. Epub 2001 Sep 11. Proc Natl Acad Sci U S A. 2001. PMID: 11553787 Free PMC article.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
-
Seeing the forest through the trees: towards a unified view on physiological calcium regulation of voltage-gated sodium channels.Biophys J. 2012 Dec 5;103(11):2243-51. doi: 10.1016/j.bpj.2012.10.020. Biophys J. 2012. PMID: 23283222 Free PMC article. Review.
Cited by
-
Worldwide Sodium Channel Conference, January 31st-February 2nd, 2024, Grindelwald, Switzerland.Bioelectricity. 2024 Dec 13;6(4):288-291. doi: 10.1089/bioe.2024.0025. eCollection 2024 Dec. Bioelectricity. 2024. PMID: 39712211
-
Nonsubstrate PI(4,5)P2 interacts with the interdomain linker to control electrochemical coupling in voltage-sensing phosphatase (VSP).Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2500651122. doi: 10.1073/pnas.2500651122. Epub 2025 Jul 29. Proc Natl Acad Sci U S A. 2025. PMID: 40729387
-
The Influence of Phosphoinositide Lipids in the Molecular Biology of Membrane Proteins: Recent Insights from Simulations.J Mol Biol. 2025 Feb 15;437(4):168937. doi: 10.1016/j.jmb.2025.168937. Epub 2025 Jan 9. J Mol Biol. 2025. PMID: 39793883 Free PMC article. Review.
References
-
- Åqvist J, Wennerström P, Nervall M, Bjelic S, Brandsdal BO. Molecular dynamics simulations of water and biomolecules with a Monte Carlo constant pressure algorithm. Chemical Physics Letters. 2004;384:288–294. doi: 10.1016/j.cplett.2003.12.039. - DOI
-
- Bauer P, Hess B, Lindahl E. GROMACS 2022.5 manual (2022.5) 2022.5Gromacs. 2023 https://manual.gromacs.org/2022.5/index.html
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

