Microneedles: A novel clinical technology for evaluating skin characteristics
- PMID: 38465749
- PMCID: PMC10926177
- DOI: 10.1111/srt.13647
Microneedles: A novel clinical technology for evaluating skin characteristics
Abstract
Background: Current methods for evaluating efficacy of cosmetics have limitations because they cannot accurately measure changes in the dermis. Skin sampling using microneedles allows identification of skin-type biomarkers, monitoring treatment for skin inflammatory diseases, and evaluating efficacy of anti-aging and anti-pigmentation products.
Materials and methods: Two studies were conducted: First, 20 participants received anti-aging treatment; second, 20 participants received anti-pigmentation treatment. Non-invasive devices measured skin aging (using high-resolution 3D-imaging in the anti-aging study) or pigmentation (using spectrophotometry in the anti-pigmentation study) at weeks 0 and 4, and adverse skin reactions were monitored. Skin samples were collected with biocompatible microneedle patches. Changes in expression of biomarkers for skin aging and pigmentation were analyzed using qRT-PCR.
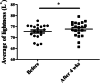
Results: No adverse events were reported. In the anti-aging study, after 4 weeks, skin roughness significantly improved in 17 out of 20 participants. qRT-PCR showed significantly increased expression of skin-aging related biomarkers: PINK1 in 16/20 participants, COL1A1 in 17/20 participants, and MSN in 16/20 participants. In the anti-pigmentation study, after 4 weeks, skin lightness significantly improved in 16/20 participants. qRT-PCR showed significantly increased expression of skin-pigmentation-related biomarkers: SOD1 in 15/20 participants and Vitamin D Receptor (VDR) in 15/20 participants. No significant change in TFAP2A was observed.
Conclusion: Skin sampling and mRNA analysis for biomarkers provides a novel, objective, quantitative method for measuring changes in the dermis and evaluating the efficacy of cosmetics. This approach complements existing evaluation methods and has potential application in assessing the effectiveness of medical devices, medications, cosmeceuticals, healthy foods, and beauty devices.
Keywords: in vivo efficacy test; skin aging; skin biomarkers; skin pigmentation.
© 2024 The Authors. Skin Research and Technology published by John Wiley & Sons Ltd.
Conflict of interest statement
Kwang Hoon Lee is the CEO of Cutis Biomedical Research Center and on the advisory committee of Raphas, a parent company of the Cutis Biomedical Research Center. Do Hyeon Jeong is the CEO of Raphas. Seo Hyeong Kim, Ji Hye Kim, Yoon Mi Choi, Su Min Seo, Eun Young Jang, and Sung Jae Lee are employees of Cutis Biomedical Research Center, and, to our knowledge, have a financial relationship with a commercial entity interested in the subject of this manuscript. The interests of these authors did not influence academic fairness in conducting this study, analyzing the results, or writing the paper.
Figures

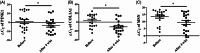
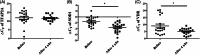
Similar articles
-
Development of a biomarker-based platform for comprehensive skin characterization using minimally invasive skin sampling and quantitative real-time PCR.Skin Res Technol. 2024 Aug;30(8):e13908. doi: 10.1111/srt.13908. Skin Res Technol. 2024. PMID: 39141418 Free PMC article.
-
In vivo evaluation of topical ascorbic acid application on skin aging by 50 MHz ultrasound.J Cosmet Dermatol. 2022 Oct;21(10):4921-4926. doi: 10.1111/jocd.14892. Epub 2022 Mar 14. J Cosmet Dermatol. 2022. PMID: 35238148
-
Acne and its post-inflammatory hyperpigmentation treatment by applying anti-acne dissolving microneedle patches.J Cosmet Dermatol. 2022 Dec;21(12):6913-6919. doi: 10.1111/jocd.15352. Epub 2022 Sep 20. J Cosmet Dermatol. 2022. PMID: 36059276 Clinical Trial.
-
Innovative cosmeceuticals: sirtuin activators and anti-glycation compounds.Semin Cutan Med Surg. 2011 Sep;30(3):163-6. doi: 10.1016/j.sder.2011.05.004. Semin Cutan Med Surg. 2011. PMID: 21925370 Review.
-
Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products.Crit Rev Food Sci Nutr. 2021;61(22):3740-3755. doi: 10.1080/10408398.2020.1805407. Epub 2020 Aug 10. Crit Rev Food Sci Nutr. 2021. PMID: 32772550 Review.
Cited by
-
Cosmeceuticals in photoaging: A review.Skin Res Technol. 2024 Sep;30(9):e13730. doi: 10.1111/srt.13730. Skin Res Technol. 2024. PMID: 39233460 Free PMC article.
-
Development of a biomarker-based platform for comprehensive skin characterization using minimally invasive skin sampling and quantitative real-time PCR.Skin Res Technol. 2024 Aug;30(8):e13908. doi: 10.1111/srt.13908. Skin Res Technol. 2024. PMID: 39141418 Free PMC article.
References
-
- Turner EO. Open‐Label assessment of the efficacy and tolerability of a skin care regimen for treating subjects with visible and physical symptoms of sensitive skin. J Cosmet Dermatol. 2022;21(9):3876‐3887. - PubMed
-
- Wang Y, Viennet C, Jeudy A, Fanian F, He L, Humbert P. Assessment of the efficacy of a new complex antisensitive skin cream. J Cosmet Dermatol. 2018;17(6):1101‐1107. - PubMed
-
- Shoshani D, Markovitz E, Monstrey SJ, Narins DJ. The modified Fitzpatrick Wrinkle Scale: a clinical validated measurement tool for nasolabial wrinkle severity assessment. Dermatol Surg. 2008;34 Suppl 1:S85‐S91; discussion S91. - PubMed
-
- Takagi Y, Shimizu M, Morokuma Y, et al. A new formula for a mild body cleanser: sodium laureth sulphate supplemented with sodium laureth carboxylate and lauryl glucoside. Int J Cosmet Sci. 2014;36(4):305‐311. - PubMed
-
- Yilmaz E, Borchert HH. Effect of lipid‐containing, positively charged nanoemulsions on skin hydration, elasticity and erythema–an in vivo study. Int J Pharm. 2006;307(2):232‐238. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

