Evaluation of the usefulness of plasma 4β-hydroxycholesterol concentration normalized by 4α-hydroxycholesterol for accurate CYP3A phenotyping
- PMID: 38465776
- PMCID: PMC10926057
- DOI: 10.1111/cts.13768
Evaluation of the usefulness of plasma 4β-hydroxycholesterol concentration normalized by 4α-hydroxycholesterol for accurate CYP3A phenotyping
Abstract
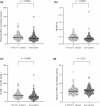
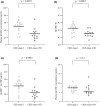
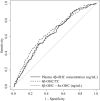
Plasma 4β-hydroxycholesterol (OHC) has drawn attention as an endogenous substrate indicating CYP3A activity. Plasma 4β-OHC is produced by hydroxylation by CYP3A4 and CYP3A5 and by cholesterol autoxidation. Plasma 4α-OHC is produced by cholesterol autoxidation and not affected by CYP3A activity. This study aimed to evaluate the usefulness of plasma 4β-OHC concentration minus plasma 4α-OHC concentration (4β-OHC-4α-OHC) compared with plasma 4β-OHC concentration and 4β-OHC/total cholesterol (TC) ratio in cross-sectional evaluation of CYP3A activity. Four hundred sixteen general adults were divided into 191 CYP3A5*1 carriers and 225 non-carriers. Twenty-six patients with chronic kidney disease (CKD) with CYP3A5*1 allele were divided into 14 with CKD stage 3 and 12 with stage 4-5D. Area under the receiver operating characteristic curve (AUC) for the three indices were evaluated for predicting presence or absence of CYP3A5*1 allele in general adults, and for predicting CKD stage 3 or stage 4-5D in patients with CKD. There was no significant difference between AUC of 4β-OHC-4α-OHC and AUC of plasma 4β-OHC concentration in general adults and in patients with CKD. AUC of 4β-OHC-4α-OHC was significantly smaller than that of 4β-OHC/TC ratio in general adults (p = 0.025), but the two indices did not differ in patients with CKD. In conclusion, in the present cross-sectional evaluation of CYP3A activity in general adults and in patients with CKD with CYP3A5*1 allele, the usefulness of 4β-OHC-4α-OHC was not different from plasma 4β-OHC concentration or 4β-OHC/TC ratio. However, because of the limitations in study design and subject selection of this research, these findings require verification in further studies.
© 2024 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zanger UM, Turpeinen M, Klein K, Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392:1093‐1108. - PubMed
-
- Hohmann N, Haefeli WE, Mikus G. CYP3A activity: towards dose adaptation to the individual. Expert Opin Drug Metab Toxicol. 2016;12:479‐497. - PubMed
-
- Bodin K, Bretillon L, Aden Y, et al. Antiepileptic drugs increase plasma levels of 4beta‐hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J Biol Chem. 2001;276:38685‐38689. - PubMed
-
- Bodin K, Andersson U, Rystedt E, et al. Metabolism of 4 beta ‐hydroxycholesterol in humans. J Biol Chem. 2002;277:31534‐31540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

