Angiotensin I and II Stimulate Cell Invasion of SARS-CoV-2: Potential Mechanism via Inhibition of ACE2 Arm of RAS
- PMID: 38466002
- PMCID: PMC11019619
- DOI: 10.33549/physiolres.935198
Angiotensin I and II Stimulate Cell Invasion of SARS-CoV-2: Potential Mechanism via Inhibition of ACE2 Arm of RAS
Abstract
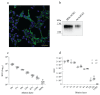
Angiotensin-converting enzyme 2 (ACE2), one of the key enzymes of the renin-angiotensin system (RAS), plays an important role in SARS-CoV-2 infection by functioning as a virus receptor. Angiotensin peptides Ang I and Ang II, the substrates of ACE2, can modulate the binding of SARS-CoV-2 Spike protein to the ACE2 receptor. In the present work, we found that co incubation of HEK-ACE2 and Vero E6 cells with the SARS-CoV-2 Spike pseudovirus (PVP) resulted in stimulation of the virus entry at low and high micromolar concentrations of Ang I and Ang II, respectively. The potency of Ang I and Ang II stimulation of virus entry corresponds to their binding affinity to ACE2 catalytic pocket with 10 times higher efficiency of Ang II. The Ang II induced mild increase of PVP infectivity at 20 microM; while at 100 microM the increase (129.74+/-3.99 %) was highly significant (p<0.001). Since the angiotensin peptides act in HEK ACE2 cells without the involvement of angiotensin type I receptors, we hypothesize that there is a steric interaction between the catalytic pocket of the ACE2 enzyme and the SARS-CoV-2 S1 binding domain. Oversaturation of the ACE2 with their angiotensin substrate might result in increased binding and entry of the SARS-CoV-2. In addition, the analysis of angiotensin peptides metabolism showed decreased ACE2 and increased ACE activity upon SARS-CoV-2 action. These effects should be taken into consideration in COVID-19 patients suffering from comorbidities such as the over-activated renin-angiotensin system as a mechanism potentially influencing the SARS-CoV-2 invasion into recipient cells.
Conflict of interest statement
Figures

Similar articles
-
Hypothesis for renin-angiotensin inhibitor mitigation of COVID-19.Med Hypotheses. 2021 Jul;152:110609. doi: 10.1016/j.mehy.2021.110609. Epub 2021 May 12. Med Hypotheses. 2021. PMID: 34048987 Free PMC article.
-
SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy.Theranostics. 2020 Jun 12;10(16):7448-7464. doi: 10.7150/thno.48076. eCollection 2020. Theranostics. 2020. PMID: 32642005 Free PMC article. Review.
-
Two hits to the renin-angiotensin system may play a key role in severe COVID-19.Kaohsiung J Med Sci. 2020 Jun;36(6):389-392. doi: 10.1002/kjm2.12237. Epub 2020 Jun 3. Kaohsiung J Med Sci. 2020. PMID: 32492292 Free PMC article. Review.
-
New Viral Diseases and New Possible Remedies by Means of the Pharmacology of the Renin-Angiotensin System.J Renin Angiotensin Aldosterone Syst. 2023 Jul 12;2023:3362391. doi: 10.1155/2023/3362391. eCollection 2023. J Renin Angiotensin Aldosterone Syst. 2023. PMID: 37476705 Free PMC article. Review.
-
Angiotensin II Promotes SARS-CoV-2 Infection via Upregulation of ACE2 in Human Bronchial Cells.Int J Mol Sci. 2022 May 4;23(9):5125. doi: 10.3390/ijms23095125. Int J Mol Sci. 2022. PMID: 35563515 Free PMC article.
References
-
- Wang K, Gheblawi M, Nikhanj A, Munan M, MacIntyre E, O’Neil C, Poglitsch M, et al. Dysregulation of ACE (Angiotensin-Converting Enzyme)-2 and Renin-Angiotensin Peptides in SARS-CoV-2 Mediated Mortality and End-Organ Injuries. Hypertension. 2022;79:365–378. doi: 10.1161/HYPERTENSIONAHA.121.18295. - DOI - PubMed
-
- Dobrocsyova V, Slamkova M, Krskova K, Balazova L, Suski M, Olszanecki R, Cacanyiova S, et al. AVE0991, a Nonpeptide Angiotensin 1–7 Receptor Agonist, Improves Glucose Metabolism in the Skeletal Muscle of Obese Zucker Rats: Possible Involvement of Prooxidant/Antioxidant Mechanisms. Oxid Med Cell Longev. 2020;2020:6372935. doi: 10.1155/2020/6372935. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
