TBCRC 057: Survey about willingness to participate in cancer clinical trials during the pandemic
- PMID: 38466037
- PMCID: PMC10926883
- DOI: 10.1002/cam4.7090
TBCRC 057: Survey about willingness to participate in cancer clinical trials during the pandemic
Abstract
Background: Breast cancer patients experienced heightened anxiety during the pandemic. Also, modifications to clinical trial activities allowing for virtual platforms, local assessments, and greater flexibility were introduced to facilitate participation. We sought to evaluate the association between pandemic-related anxiety and willingness to participate in trials and how pandemic-era modifications to trial activities affect the decision to participate.
Methods: We conducted an online survey from August to September, 2021 of patients with breast cancer assessing pandemic-related anxiety; clinical trials knowledge and attitudes; willingness to participate during and before the pandemic; and how each modification affects the decision to participate. Fisher's exact tests evaluated differences in proportions and two-sample t-tests evaluated differences in means. The association of pandemic-related anxiety with a decline in willingness to participate during compared to prior to the pandemic was modeled using logistic regression.
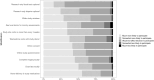
Results: Among 385 respondents who completed the survey, 81% reported moderate-severe pandemic-related anxiety. Mean willingness to participate in a trial was lower during the pandemic than prior [2.97 (SD 1.17) vs. 3.10 (SD 1.09), (p < 0.001)]. Severe anxiety was associated with higher odds of diminished willingness to participate during the pandemic compared to prior (OR 5.07). Each of the modifications, with the exception of opting out of research-only blood tests, were endorsed by >50% of respondents as strategies that would increase their likelihood of deciding to participate.
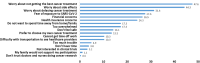
Conclusions: While pandemic-related anxiety was associated with diminished willingness to participate in trials, the leading reasons for reluctance to consider trial participation were unrelated to the pandemic but included worries about not getting the best treatment, side effects, and delaying care. Patients view trial modifications favorably, supporting continuation of these modifications, as endorsed by the National Cancer Institute and others.
Keywords: COVID-19; breast cancer; clinical trials; pandemic; survey.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Smith receiving research grants to institution from Pfizer, spouse has stock in Abbvie and Abbott Labs, and is currently employed at AstraZeneca. Dr. Melisko receives research funding from OBI Pharma, Daehwa, and Novartis. Dr. Rocque received research funding from Genentech, Pfizer, and Carevive and consulting fees for Genentech and Pfizer.
Figures


References
-
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784‐1792. - PubMed
-
- Gradishar WJ, Moran MS, Abraham J, et al. NCCN guidelines(R) insights: breast cancer, version 4.2021. J Natl Compr Cancer Netw. 2021;19(5):484‐493. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

