Efficacy of a novel multi-enzyme feed additive on growth performance, nutrient digestibility, and gut microbiome of weanling pigs fed corn-wheat or wheat-barley-based diet
- PMID: 38466229
- PMCID: PMC10977034
- DOI: 10.1093/jas/skae064
Efficacy of a novel multi-enzyme feed additive on growth performance, nutrient digestibility, and gut microbiome of weanling pigs fed corn-wheat or wheat-barley-based diet
Abstract
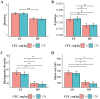
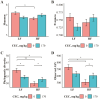
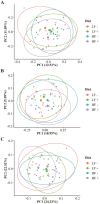
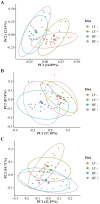
One-hundred-and-ninety-two weanling pigs (6.7 kg body weight) were used to evaluate the impact of a carbohydrases-protease enzyme complex (CPEC) on growth performance, nutrient digestibility, and gut microbiome. Pigs were assigned to one of the four dietary treatments for 42 d according to a 2 × 2 factorial arrangement of diet type (low fiber [LF] or high fiber [HF]) and CPEC supplementation (0 or 170 mg/kg diet). The LF diet was prepared as corn-wheat-based diet while the HF diet was wheat-barley-based and contained wheat middlings and canola meal. Each dietary treatment consisted of 12 replicate pens (six replicates per gender) and four pigs per replicate pen. Over the 42-d period, there was no interaction between diet type and CPEC supplementation on growth performance indices of pigs. Dietary addition of CPEC improved (P < 0.05) the body weight of pigs at days 28 and 42 and the gain-to-feed ratio of pigs from days 0 to 14. During the entire experimental period, dietary CPEC supplementation improved (P < 0.05) the average daily gain and gain-to-feed ratio of pigs. There were interactions between diet type and CPEC supplementation on apparent total tract digestibility (ATTD) of dry matter (DM; P < 0.01), gross energy (GE; P < 0.01), and neutral detergent fiber (NDF; P < 0.05) at d 42. Dietary CPEC addition improved (P < 0.05) ATTD of DM, GE, and NDF in the HF diets. At day 43, dietary CPEC addition resulted in improved (P < 0.05) apparent ileal digestibility (AID) of NDF and interactions (P < 0.05) between diet type and CPEC supplementation on AID of DM and crude fiber. Alpha diversity indices including phylogenetic diversity and observed amplicon sequence variants of fecal microbiome increased (P < 0.05) by the addition of CPEC to the HF diets on day 42. An interaction (P < 0.05) between diet type and CPEC addition on Bray-Curtis dissimilarity index and Unweighted UniFrac distances was observed on day 42. In conclusion, CPEC improved weanling pig performance and feed efficiency, especially in wheat-barley diets, while dietary fiber composition had a more significant impact on fecal microbial communities than CPEC administration. The results of this study underscores carbohydrase's potential to boost pig performance without major microbiome changes.
Keywords: carbohydrase; fiber; growth performance; gut microbiome; weanling pigs.
Plain language summary
There is a pressing need to enhance livestock production efficiency to meet the growing global demand for meat. Carbohydrases and proteases are enzymes typically added to swine diets to improve nutrient utilization, leading to better growth rates and feed efficiency. This ultimately contributes to sustainable and economically viable pig farming. However, more research is required to better understand how carbohydrases and proteases interact with different diet types to optimize dietary formulations, and how this may influence gut microbiome composition. In this study, 192 weaner pigs (~7 kg) were assigned to a low-fiber diet or a high-fiber diet. Each diet type was with or without a carbohydrases and protease multi-enzyme supplementation. The results showed that adding a multi-enzyme combination to the pigs’ diet significantly improved the pig’s performance, regardless of diet type. Improvement in nutrient digestibility was more pronounced in pigs fed the high-fiber diet and that dietary fiber had a greater influence on the composition of fecal microbes. In essence, the study demonstrates that the multi-enzyme can boost pig growth and feed efficiency in diets with varying fiber complexity without causing significant changes in their gut microbiome.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society of Animal Science.
Conflict of interest statement
Deepak Velayudhan and Ester Vinyeta are employees of Danisco Animal Nutrition and Health (IFF), which provided financial support for this study. Other authors disclose that there are no conflicts of interest.
Figures
Similar articles
-
Effects of a fiber-degrading enzyme on ileal digestibility of amino acids and fiber and total tract digestibility of energy and fiber in growing pigs fed diets with high level of corn distillers grains with solubles.J Anim Sci. 2025 Jan 4;103:skaf076. doi: 10.1093/jas/skaf076. J Anim Sci. 2025. PMID: 40096523
-
Evaluation of multienzyme supplementation and fiber levels on nutrient and energy digestibility of diets fed to gestating sows and growing pigs.J Anim Sci. 2023 Jan 3;101:skad375. doi: 10.1093/jas/skad375. J Anim Sci. 2023. PMID: 37936246 Free PMC article.
-
Unveiling the influence of adaptation time on xylanase and arabinoxylan-oligosaccharide efficacy: a study on nutrient digestibility, viscosity, and scanning electron microscopy in the small and large intestine of growing pigs fed insoluble fiber.J Anim Sci. 2024 Jan 3;102:skad378. doi: 10.1093/jas/skad378. J Anim Sci. 2024. PMID: 37991108 Free PMC article.
-
Swine Gastrointestinal Microbiota and the Effects of Dietary Amino Acids on Its Composition and Metabolism.Int J Mol Sci. 2024 Jan 19;25(2):1237. doi: 10.3390/ijms25021237. Int J Mol Sci. 2024. PMID: 38279233 Free PMC article. Review.
-
Unconventional plant sources as alternative feedstuffs in broiler rabbit nutrition: a scoping review.Trop Anim Health Prod. 2025 Mar 19;57(3):135. doi: 10.1007/s11250-025-04393-9. Trop Anim Health Prod. 2025. PMID: 40106044
Cited by
-
Impacts of Bacillus-based biotics and an enzyme cocktail on growth performance, immunity, and gut pathogenic microorganisms of nursery pigs under commercial conditions.Front Vet Sci. 2025 Jul 25;12:1627739. doi: 10.3389/fvets.2025.1627739. eCollection 2025. Front Vet Sci. 2025. PMID: 40786974 Free PMC article.
-
Bacillus spp. as potential probiotics: promoting piglet growth by improving intestinal health.Front Vet Sci. 2024 Jul 26;11:1429233. doi: 10.3389/fvets.2024.1429233. eCollection 2024. Front Vet Sci. 2024. PMID: 39132437 Free PMC article. Review.
-
Effects of Extrusion on the Available Energy and Nutrient Digestibility of Wheat and Its Application in Weaned Piglets.Animals (Basel). 2025 Feb 12;15(4):528. doi: 10.3390/ani15040528. Animals (Basel). 2025. PMID: 40003011 Free PMC article.
References
-
- Agyekum, A. K., Sands J. S., Regassa A., Kiarie E., Weihrauch D., Kim W. K., and Nyachoti C. M... 2015. Effect of supplementing a fibrous diet with a xylanase and β-glucanase blend on growth performance, intestinal glucose uptake, and transport-associated gene expression in growing pigs. J. Anim. Sci. 93:3483–3493. doi: 10.2527/jas.2015-9027 - DOI - PubMed
-
- AOAC. 2000. Official methods of analysis. 17th ed.Arlington (VA): Association of Official Analytical Chemists.
-
- AOAC. 2006. Official methods of analysis. 18th ed.Arlington (VA): Association of Official Analytical Chemists.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

