Opioid Prescribing and Outcomes in Patients With Sickle Cell Disease Post-2016 CDC Guideline
- PMID: 38466269
- PMCID: PMC10928539
- DOI: 10.1001/jamainternmed.2023.8538
Opioid Prescribing and Outcomes in Patients With Sickle Cell Disease Post-2016 CDC Guideline
Erratum in
-
Errors in Key Points, Abstract, Text, Figures, Table 2, and Supplement 1.JAMA Intern Med. 2025 Apr 1;185(4):479. doi: 10.1001/jamainternmed.2024.8314. JAMA Intern Med. 2025. PMID: 39928342 Free PMC article. No abstract available.
Abstract
Importance: Although the intention of the 2016 US Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain was not to limit pain treatment for patients with sickle cell disease (SCD), clinicians and patients have recognized the possibility that the guideline may have altered outcomes for this population. However, the outcomes of the 2016 guideline for this patient population are unknown.
Objective: To examine changes in opioid prescribing patterns and health outcomes among patients with SCD before and after the release of the 2016 CDC guideline.
Design, setting, and participants: This retrospective cohort study conducted interrupted time series analysis of claims data from the Merative MarketScan Commercial Database from January 1, 2011, to December 31, 2019. In this population-based study in the US, individuals with SCD who were at least 1 year of age, had no cancer diagnosis, and had pharmacy coverage for the month of measurement were included. The data were analyzed from January 2021 to November 2023.
Exposure: The CDC Guideline for Prescribing Opioids for Chronic Pain released in March 2016.
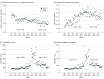
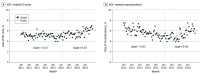
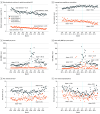
Main outcomes and measures: The main variables measured in this study included the practice of opioid prescribing among patients with SCD (ie, rate of opioid prescriptions dispensed, mean number of days supplied, mean total morphine milligram equivalents [MME] per patient, and mean daily MME per opioid prescription) and pain-related health outcomes (rates of emergency department visits related to vaso-occlusive crises [VOC] and hospitalizations related to VOC).
Results: The cohort included 14 979 patients with SCD (mean [SD] age, 25.9 [16.9] years; 8520 [56.9%] female). Compared with the preguideline trends, the following changes were observed after the guideline was released: significant decreases in the coefficient for change in slope of the opioid dispensing rate (-0.29 [95% CI, -0.39 to -0.20] prescriptions per 100 person-month; P < .001), the number of days supplied per prescription (-0.05 [95% CI, -0.06 to -0.04] days per prescription-month; P < .001), and opioid dosage (-141.0 [95% CI, -219.5 to -62.5] MME per person-month; P = .001; -10.1 [95% CI, -14.6 to -5.6] MME/prescription-month; P < .001). Conversely, a significant increase in VOC-related hospitalizations occurred after the guideline release (0.16 [95% CI, 0.07-0.25] hospitalizations per 100 person-month; P = .001). These changes were observed to a greater extent among adult patients, but pediatric patients experienced similar changes in several measures, even though the guideline focused exclusively on adult patients.
Conclusions and relevance: This retrospective cohort study showed that the 2016 CDC guideline may have had unintended negative outcomes on the patient population living with SCD.
Conflict of interest statement
Figures
References
-
- Togun AT, Mandic PK, Wurtz R, Jeffery MM, Beebe T. Association of opioid fills with Centers for Disease Control and Prevention opioid guidelines and payer coverage policies: physician, insurance and geographic factors. Int J Clin Pharm. 2022;44(2):428-438. doi:10.1007/s11096-021-01360-w - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

