Emerging Cerebrospinal Fluid Biomarkers of Disease Activity and Progression in Multiple Sclerosis
- PMID: 38466277
- PMCID: PMC10928543
- DOI: 10.1001/jamaneurol.2024.0017
Emerging Cerebrospinal Fluid Biomarkers of Disease Activity and Progression in Multiple Sclerosis
Abstract
Importance: Biomarkers distinguishing nonrelapsing progressive disease biology from relapsing biology in multiple sclerosis (MS) are lacking. Cerebrospinal fluid (CSF) is an accessible fluid that most closely reflects central nervous system biology.
Objective: To identify CSF biological measures associated with progressive MS pathobiology.
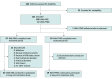
Design, setting, and participants: This cohort study assessed data from 2 prospective MS cohorts: a test cohort provided serial CSF, clinical, and imaging assessments in a multicenter study of patients with relapsing MS (RMS) or primary progressive MS (PPMS) who were initiating anti-CD20 treatment (recruitment: 2016-2018; analysis: 2020-2023). A single-site confirmation cohort was used to assess CSF at baseline and long-term (>10 year) clinical follow-up (analysis: 2022-2023).
Exposures: Test-cohort participants initiated standard-of-care ocrelizumab treatment. Confirmation-cohort participants were untreated or received standard-of-care disease-modifying MS therapies.
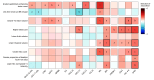
Main outcomes and measures: Twenty-five CSF markers, including neurofilament light chain, neurofilament heavy chain, and glial fibrillary acid protein (GFAP); 24-week confirmed disability progression (CDP24); and brain magnetic resonance imaging measures reflecting focal injury, tissue loss, and progressive biology (slowly expanding lesions [SELs]).
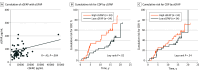
Results: The test cohort (n = 131) included 100 patients with RMS (mean [SD] age, 36.6 [10.4] years; 68 [68%] female and 32 [32%] male; Expanded Disability Status Scale [EDSS] score, 0-5.5), and 31 patients with PPMS (mean [SD] age, 44.9 [7.4] years; 15 [48%] female and 16 [52%] male; EDSS score, 3.0-6.5). The confirmation cohort (n = 68) included 41 patients with RMS and 27 with PPMS enrolled at diagnosis (age, 40 years [range, 20-61 years]; 47 [69%] female and 21 [31%] male). In the test cohort, GFAP was correlated with SEL count (r = 0.33), greater proportion of T2 lesion volume from SELs (r = 0.24), and lower T1-weighted intensity within SELs (r = -0.33) but not with acute inflammatory measures. Neurofilament heavy chain was correlated with SEL count (r = 0.25) and lower T1-weighted intensity within SELs (r = -0.28). Immune markers correlated with measures of acute inflammation and, unlike GFAP, were impacted by anti-CD20. In the confirmation cohort, higher baseline CSF GFAP levels were associated with long-term CDP24 (hazard ratio, 2.1; 95% CI, 1.3-3.4; P = .002).
Conclusions and relevance: In this study, activated glial markers (in particular GFAP) and neurofilament heavy chain were associated specifically with nonrelapsing progressive disease outcomes (independent of acute inflammatory activity). Elevated CSF GFAP was associated with long-term MS disease progression.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

