Recent Progress in Improving Rate Performance of Cellulose-Derived Carbon Materials for Sodium-Ion Batteries
- PMID: 38466498
- PMCID: PMC10928064
- DOI: 10.1007/s40820-024-01351-2
Recent Progress in Improving Rate Performance of Cellulose-Derived Carbon Materials for Sodium-Ion Batteries
Abstract
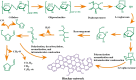
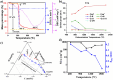
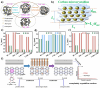
Cellulose-derived carbon is regarded as one of the most promising candidates for high-performance anode materials in sodium-ion batteries; however, its poor rate performance at higher current density remains a challenge to achieve high power density sodium-ion batteries. The present review comprehensively elucidates the structural characteristics of cellulose-based materials and cellulose-derived carbon materials, explores the limitations in enhancing rate performance arising from ion diffusion and electronic transfer at the level of cellulose-derived carbon materials, and proposes corresponding strategies to improve rate performance targeted at various precursors of cellulose-based materials. This review also presents an update on recent progress in cellulose-based materials and cellulose-derived carbon materials, with particular focuses on their molecular, crystalline, and aggregation structures. Furthermore, the relationship between storage sodium and rate performance the carbon materials is elucidated through theoretical calculations and characterization analyses. Finally, future perspectives regarding challenges and opportunities in the research field of cellulose-derived carbon anodes are briefly highlighted.
Keywords: Anode materials; Cellulose; Hard carbon; Rate performance; Sodium-ion batteries.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures












References
-
- T. He, X. Kang, F. Wang, J. Zhang, T. Zhang et al., Capacitive contribution matters in facilitating high power battery materials toward fast-charging alkali metal ion batteries. Mater. Sci. Eng. R. Rep. 154, 100737 (2023). 10.1016/j.mser.2023.100737 - DOI
-
- Y. Wan, Y. Liu, D. Chao, W. Li, D. Zhao, Recent advances in hard carbon anodes with high initial Coulombic efficiency for sodium-ion batteries. Nano Mater. Sci. 5, 189–201 (2023). 10.1016/j.nanoms.2022.02.001 - DOI
Publication types
LinkOut - more resources
Full Text Sources
