Survival-Associated Cellular Response Maintained in Pancreatic Ductal Adenocarcinoma (PDAC) Switched Between Soft and Stiff 3D Microgel Culture
- PMID: 38466617
- PMCID: PMC11005012
- DOI: 10.1021/acsbiomaterials.3c01079
Survival-Associated Cellular Response Maintained in Pancreatic Ductal Adenocarcinoma (PDAC) Switched Between Soft and Stiff 3D Microgel Culture
Abstract
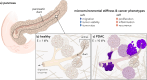
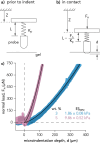
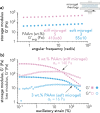
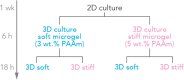
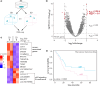
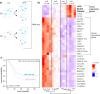
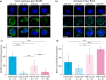
Pancreatic ductal adenocarcinoma (PDAC) accounts for about 90% of all pancreatic cancer cases. Five-year survival rates have remained below 12% since the 1970s, in part due to the difficulty in detection prior to metastasis (migration and invasion into neighboring organs and glands). Mechanical memory is a concept that has emerged over the past decade that may provide a path toward understanding how invading PDAC cells "remember" the mechanical properties of their diseased ("stiff", elastic modulus, E ≈ 10 kPa) microenvironment even while invading a healthy ("soft", E ≈ 1 kPa) microenvironment. Here, we investigated the role of mechanical priming by culturing a dilute suspension of PDAC (FG) cells within a 3D, rheologically tunable microgel platform from hydrogels with tunable mechanical properties. We conducted a suite of acute (short-term) priming studies where we cultured PDAC cells in either a soft (E ≈ 1 kPa) or stiff (E ≈ 10 kPa) environment for 6 h, then removed and placed them into a new soft or stiff 3D environment for another 18 h. Following these steps, we conducted RNA-seq analyses to quantify gene expression. Initial priming in the 3D culture showed persistent gene expression for the duration of the study, regardless of the subsequent environments (stiff or soft). Stiff 3D culture was associated with the downregulation of tumor suppressors (LATS1, BCAR3, CDKN2C), as well as the upregulation of cancer-associated genes (RAC3). Immunofluorescence staining (BCAR3, RAC3) further supported the persistence of this cellular response, with BCAR3 upregulated in soft culture and RAC3 upregulated in stiff-primed culture. Stiff-primed genes were stratified against patient data found in The Cancer Genome Atlas (TCGA). Upregulated genes in stiff-primed 3D culture were associated with decreased survival in patient data, suggesting a link between patient survival and mechanical priming.
Keywords: 3D cell culture; confinement; mechanical memory; microgel; stiffness.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Piezo1 Mediates Glycolysis-Boosted Pancreatic Ductal Adenocarcinoma Chemoresistance within a Biomimetic Three-Dimensional Matrix Stiffness.ACS Biomater Sci Eng. 2024 Dec 9;10(12):7632-7646. doi: 10.1021/acsbiomaterials.4c01319. Epub 2024 Nov 18. ACS Biomater Sci Eng. 2024. PMID: 39556518
-
Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties.Acta Biomater. 2018 Feb;67:331-340. doi: 10.1016/j.actbio.2017.11.037. Epub 2017 Dec 2. Acta Biomater. 2018. PMID: 29191507 Free PMC article.
-
Viscoelastic stiffening of gelatin hydrogels for dynamic culture of pancreatic cancer spheroids.Acta Biomater. 2024 Mar 15;177:203-215. doi: 10.1016/j.actbio.2024.02.010. Epub 2024 Feb 12. Acta Biomater. 2024. PMID: 38354874 Free PMC article.
-
Designer hydrogels: Shedding light on the physical chemistry of the pancreatic cancer microenvironment.Cancer Lett. 2018 Nov 1;436:22-27. doi: 10.1016/j.canlet.2018.08.008. Epub 2018 Aug 14. Cancer Lett. 2018. PMID: 30118843 Free PMC article. Review.
-
The role of epithelial-mesenchymal transition and autophagy in pancreatic ductal adenocarcinoma invasion.Cell Death Dis. 2023 Aug 7;14(8):506. doi: 10.1038/s41419-023-06032-3. Cell Death Dis. 2023. PMID: 37550301 Free PMC article. Review.
Cited by
-
Differential Effects of Confinement on the Dynamics of Normal and Tumor-Derived Pancreatic Ductal Organoids.ACS Appl Bio Mater. 2024 Dec 16;7(12):8489-8502. doi: 10.1021/acsabm.4c01301. Epub 2024 Nov 22. ACS Appl Bio Mater. 2024. PMID: 39576883 Free PMC article.
-
Stress relaxation rates of myocardium from failing and non-failing hearts.Biomech Model Mechanobiol. 2025 Feb;24(1):265-280. doi: 10.1007/s10237-024-01909-4. Epub 2024 Dec 31. Biomech Model Mechanobiol. 2025. PMID: 39741200 Free PMC article.
-
Single-cell transcriptional dissection illuminates an evolution of immunosuppressive microenvironment during pancreatic ductal adenocarcinoma metastasis.Signal Transduct Target Ther. 2025 Jun 9;10(1):182. doi: 10.1038/s41392-025-02265-0. Signal Transduct Target Ther. 2025. PMID: 40484878 Free PMC article.
-
Prior Fc receptor activation primes macrophages for increased sensitivity to IgG via long-term and short-term mechanisms.Dev Cell. 2024 Nov 4;59(21):2882-2896.e7. doi: 10.1016/j.devcel.2024.07.017. Epub 2024 Aug 12. Dev Cell. 2024. PMID: 39137774
-
Sp1 mechanotransduction regulates breast cancer cell invasion in response to multiple tumor-mimicking extracellular matrix cues.bioRxiv [Preprint]. 2025 Mar 19:2025.03.18.643983. doi: 10.1101/2025.03.18.643983. bioRxiv. 2025. PMID: 40166320 Free PMC article. Preprint.
References
-
- Howlader N.; Noone A.; Krapcho M.; Miller D.; Brest A.; Yu M.; Ruhl J.; Tatalovich Z.; Mariotto A.; Lewis D.; Chen H.; Feuer E.; Cronin K.. SEER Cancer Statistics Review, 1975–2017; National Cancer Institute. Bethesda, MD, based on November 2019 SEER data submission, posted to the SEER web site, April 2020, https://seer.cancer.gov/csr/1975_2017/. (accessed: 08-03-2023).
-
- Survival Rates for Pancreatic Cancer, 2023. https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosi.... (accessed: 08-03-2023).
-
- Konstantinidis I. T.; Warshaw A. L.; Allen J. N.; Blaszkowsky L. S.; Castillo C. F.-d.; Deshpande V.; Hong T. S.; Kwak E. L.; Lauwers G. Y.; Ryan D. P.; Wargo J. A.; Lillemoe K. D.; Ferrone C. R. Pancreatic Ductal Adenocarcinoma: Is There a Survival Difference for R1 Resections Versus Locally Advanced Unresectable Tumors? What Is a “True” R0 Resection?. Ann. Surg. 2013, 257, 731–736. 10.1097/sla.0b013e318263da2f. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
