Hybrid Immunity and SARS-CoV-2 Antibodies: Results of the HEROES-RECOVER Prospective Cohort Study
- PMID: 38466720
- PMCID: PMC12097991
- DOI: 10.1093/cid/ciae130
Hybrid Immunity and SARS-CoV-2 Antibodies: Results of the HEROES-RECOVER Prospective Cohort Study
Abstract
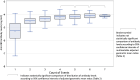
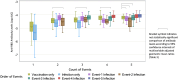
Background: There are limited data on whether hybrid immunity differs by count and order of immunity-conferring events (infection with severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] or vaccination against coronavirus disease 2019 [COVID-19]). From a multi-site cohort of frontline workers, we examined the heterogeneity of the effect of hybrid immunity on SARS-CoV-2 antibody levels.
Methods: Exposures included event count and event order, categorized into 7 permutations. Outcome was level of serum antibodies against receptor-binding domain (RBD) of the ancestral SARS-CoV-2 spike protein (total RBD-binding immunoglobulin). Means were examined up to 365 days after each of the first to seventh events.
Results: Analysis included 5793 participants measured from 7 August 2020 to 15 April 2023. Hybrid immunity from infection before 1 or 2 vaccine doses elicited modestly superior antibody responses after the second and third events (compared with infections or vaccine doses alone). This superiority was not repeated after additional events. Among adults infected before vaccination, adjusted geometric mean ratios (95% confidence interval [CI]) of anti-RBD early response (versus vaccinated only) were 1.23 (1.14-1.33), 1.09 (1.03-1.14), 0.87 (.81-.94), and 0.99 (.85-1.15) after the second to fifth events, respectively. Post-vaccination infections elicited superior responses; adjusted geometric mean ratios (95% CI) of anti-RBD early response (versus vaccinated only) were 0.93 (.75-1.17), 1.11 (1.06-1.16), 1.17 (1.11-1.24), and 1.20 (1.07-1.34) after the second to fifth events, respectively.
Conclusions: Evidence of heterogeneity in antibody levels by permutations of infection and vaccination history could inform COVID-19 vaccination policy.
Keywords: COVID-19; SARS-CoV-2; antibody durability; hybrid immunity; mRNA vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. A. L. N. reports research funding from Pfizer and Vir Biotechnology for unrelated studies. M. G. reports grants from CDC, Abt Associates, Westat, and Vanderbilt University Medical Center and participation on the Texas Pediatric Society, Texas Chapter of the American Academy of Pediatrics Infectious Diseases and Immunization Committee as co-chair. J. L. U. reports patent 16/900 798. R. S. reports consulting fees for the California legal case Ebers v. Castle Park, payment for lectures and presentations from the American Council of Life Insurers and 360 Dx/Beckman Coulter, patents 17/269 092 and 706843, and stock options for Geneticture, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

