Autocrine phosphatase PDP2 inhibits ferroptosis by dephosphorylating ACSL4 in the Luminal A Breast Cancer
- PMID: 38466744
- PMCID: PMC10927110
- DOI: 10.1371/journal.pone.0299571
Autocrine phosphatase PDP2 inhibits ferroptosis by dephosphorylating ACSL4 in the Luminal A Breast Cancer
Abstract
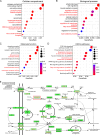
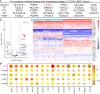
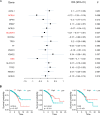
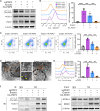
Phosphatases can dephosphorylate phosphorylated kinases, leading to their inactivation, and ferroptosis is a type of cell death. Therefore, our aim is to identify phosphatases associated with ferroptosis by analyzing the differentially expressed genes (DEGs) of the Luminal A Breast Cancer (LumABC) cohort from the Cancer Genome Atlas (TCGA). An analysis of 260 phosphatase genes from the GeneCard database revealed that out of the 28 DEGs with high expression, only the expression of pyruvate dehydrogenase phosphatase 2 (PDP2) had a significant correlation with patient survival. In addition, an analysis of DEGs using gene ontology, Kyoto Encyclopedia of Genes and Genomes and gene set enrichment analysis revealed a significant variation in the expression of ferroptosis-related genes. To further investigate this, we analyzed 34 ferroptosis-related genes from the TCGA-LumABC cohort. The expression of long-chain acyl-CoA synthetase 4 (ACSL4) was found to have the highest correlation with the expression of PDP2, and its expression was also inversely proportional to the survival rate of patients. Western blot experiments using the MCF-7 cell line showed that the phosphorylation level of ACSL4 was significantly lower in cells transfected with the HA-PDP2 plasmid, and ferroptosis was correspondingly reduced (p < 0.001), as indicated by data from flow cytometry detection of membrane-permeability cell death stained with 7-aminoactinomycin, lipid peroxidation, and Fe2+. Immunoprecipitation experiments further revealed that the phosphorylation level of ACSL4 was only significantly reduced in cells where PDP2 and ACSL4 co-precipitated. These findings suggest that PDP2 may act as a phosphatase to dephosphorylate and inhibit the activity of ACSL4, which had been phosphorylated and activated in LumABC cells. Further experiments are needed to confirm the molecular mechanism of PDP2 inhibiting ferroptosis.
Copyright: © 2024 Zhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Deciphering heart failure: an integrated proteomic and transcriptomic approach with experimental validation.Funct Integr Genomics. 2024 Oct 23;24(6):196. doi: 10.1007/s10142-024-01475-z. Funct Integr Genomics. 2024. PMID: 39441209
-
Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy.EBioMedicine. 2021 Sep;71:103560. doi: 10.1016/j.ebiom.2021.103560. Epub 2021 Sep 2. EBioMedicine. 2021. PMID: 34482070 Free PMC article.
-
High-fat diet impairs ferroptosis and promotes cancer invasiveness via downregulating tumor suppressor ACSL4 in lung adenocarcinoma.Biol Direct. 2021 May 31;16(1):10. doi: 10.1186/s13062-021-00294-7. Biol Direct. 2021. PMID: 34053456 Free PMC article.
-
ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries.Int J Mol Sci. 2023 Jun 12;24(12):10021. doi: 10.3390/ijms241210021. Int J Mol Sci. 2023. PMID: 37373168 Free PMC article. Review.
-
Acyl-CoA synthase ACSL4: an essential target in ferroptosis and fatty acid metabolism.Chin Med J (Engl). 2023 Nov 5;136(21):2521-2537. doi: 10.1097/CM9.0000000000002533. Chin Med J (Engl). 2023. PMID: 37442770 Free PMC article. Review.
Cited by
-
Protein serine/threonine phosphatases in tumor microenvironment: a vital player and a promising therapeutic target.Theranostics. 2025 Jan 1;15(3):1164-1184. doi: 10.7150/thno.104529. eCollection 2025. Theranostics. 2025. PMID: 39776803 Free PMC article. Review.
-
Mitochondrial pyruvate dehydrogenase phosphatase metabolism disorder in malignant tumors.Oncol Res. 2025 Jul 18;33(8):1861-1874. doi: 10.32604/or.2025.063716. eCollection 2025. Oncol Res. 2025. PMID: 40746885 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

