A negative feedback loop is critical for recovery of RpoS after stress in Escherichia coli
- PMID: 38466775
- PMCID: PMC10957080
- DOI: 10.1371/journal.pgen.1011059
A negative feedback loop is critical for recovery of RpoS after stress in Escherichia coli
Abstract
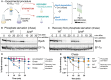
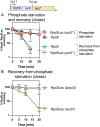
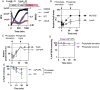
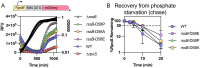
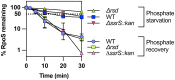
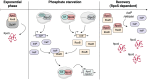
RpoS is an alternative sigma factor needed for the induction of the general stress response in many gammaproteobacteria. Tight regulation of RpoS levels and activity is required for bacterial growth and survival under stress. In Escherichia coli, various stresses lead to higher levels of RpoS due to increased translation and decreased degradation. During non-stress conditions, RpoS is unstable, because the adaptor protein RssB delivers RpoS to the ClpXP protease. RpoS degradation is prevented during stress by the sequestration of RssB by anti-adaptors, each of which is induced in response to specific stresses. Here, we examined how the stabilization of RpoS is reversed during recovery of the cell from stress. We found that RpoS degradation quickly resumes after recovery from phosphate starvation, carbon starvation, and when transitioning from stationary phase back to exponential phase. This process is in part mediated by the anti-adaptor IraP, known to promote RpoS stabilization during phosphate starvation via the sequestration of adaptor RssB. The rapid recovery from phosphate starvation is dependent upon a feedback loop in which RpoS transcription of rssB, encoding the adaptor protein, plays a critical role. Crl, an activator of RpoS that specifically binds to and stabilizes the complex between the RNA polymerase and RpoS, is also required for the feedback loop to function efficiently, highlighting a critical role for Crl in restoring RpoS basal levels.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Update of
-
A negative feedback loop is critical for recovery of RpoS after stress in Escherichia coli.bioRxiv [Preprint]. 2023 Nov 10:2023.11.09.566509. doi: 10.1101/2023.11.09.566509. bioRxiv. 2023. Update in: PLoS Genet. 2024 Mar 11;20(3):e1011059. doi: 10.1371/journal.pgen.1011059. PMID: 38077006 Free PMC article. Updated. Preprint.
Similar articles
-
A negative feedback loop is critical for recovery of RpoS after stress in Escherichia coli.bioRxiv [Preprint]. 2023 Nov 10:2023.11.09.566509. doi: 10.1101/2023.11.09.566509. bioRxiv. 2023. Update in: PLoS Genet. 2024 Mar 11;20(3):e1011059. doi: 10.1371/journal.pgen.1011059. PMID: 38077006 Free PMC article. Updated. Preprint.
-
Modulating RssB activity: IraP, a novel regulator of sigma(S) stability in Escherichia coli.Genes Dev. 2006 Apr 1;20(7):884-97. doi: 10.1101/gad.1400306. Genes Dev. 2006. PMID: 16600914 Free PMC article.
-
IraL is an RssB anti-adaptor that stabilizes RpoS during logarithmic phase growth in Escherichia coli and Shigella.mBio. 2014 May 27;5(3):e01043-14. doi: 10.1128/mBio.01043-14. mBio. 2014. PMID: 24865554 Free PMC article.
-
The two-component network and the general stress sigma factor RpoS (sigma S) in Escherichia coli.Adv Exp Med Biol. 2008;631:40-53. doi: 10.1007/978-0-387-78885-2_4. Adv Exp Med Biol. 2008. PMID: 18792681 Review.
-
Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase.Microbiol Mol Biol Rev. 2002 Sep;66(3):373-95, table of contents. doi: 10.1128/MMBR.66.3.373-395.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208995 Free PMC article. Review.
Cited by
-
Positive feedback regulation between RpoS and BosR in the Lyme disease pathogen.mBio. 2025 Mar 12;16(3):e0276624. doi: 10.1128/mbio.02766-24. Epub 2025 Jan 28. mBio. 2025. PMID: 39873484 Free PMC article.
-
Positive feedback regulation between RpoS and BosR in the Lyme disease pathogen.bioRxiv [Preprint]. 2024 Sep 15:2024.09.14.613071. doi: 10.1101/2024.09.14.613071. bioRxiv. 2024. Update in: mBio. 2025 Mar 12;16(3):e0276624. doi: 10.1128/mbio.02766-24. PMID: 39314342 Free PMC article. Updated. Preprint.
-
Deciphering the proteome of Escherichia coli K-12: Integrating transcriptomics and machine learning to annotate hypothetical proteins.Comput Struct Biotechnol J. 2025 Jul 24;27:3565-3578. doi: 10.1016/j.csbj.2025.07.036. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40821719 Free PMC article.
-
RpoS and the bacterial general stress response.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0015122. doi: 10.1128/mmbr.00151-22. Epub 2024 Feb 27. Microbiol Mol Biol Rev. 2024. PMID: 38411096 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

