Nebulization and In Vitro Upper Airway Deposition of Liposomal Carrier Systems
- PMID: 38466817
- PMCID: PMC10988550
- DOI: 10.1021/acs.molpharmaceut.3c01146
Nebulization and In Vitro Upper Airway Deposition of Liposomal Carrier Systems
Abstract
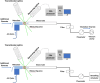
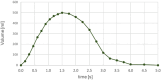
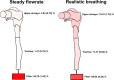
Liposomal carrier systems have emerged as a promising technology for pulmonary drug delivery. This study focuses on two selected liposomal systems, namely, dipalmitoylphosphatidylcholine stabilized by phosphatidic acid and cholesterol (DPPC-PA-Chol) and dipalmitoylphosphatidylcholine stabilized by polyethylene glycol and cholesterol (DPPC-PEG-Chol). First, the research investigates the stability of these liposomal systems during the atomization process using different kinds of nebulizers (air-jet, vibrating mesh, and ultrasonic). The study further explores the aerodynamic particle size distribution of the aerosol generated by the nebulizers. The nebulizer that demonstrated optimal stability and particle size was selected for more detailed investigation, including Andersen cascade impactor measurements, an assessment of the influence of flow rate and breathing profiles on aerosol particle size, and an in vitro deposition study on a realistic replica of the upper airways. The most suitable combination of a nebulizer and liposomal system was DPPC-PA-Chol nebulized by a Pari LC Sprint Star in terms of stability and particle size. The influence of the inspiration flow rate on the particle size was not very strong but was not negligible either (decrease of Dv50 by 1.34 μm with the flow rate increase from 8 to 60 L/min). A similar effect was observed for realistic transient inhalation. According to the in vitro deposition measurement, approximately 90% and 70% of the aerosol penetrated downstream of the trachea using the stationary flow rate and the realistic breathing profile, respectively. These data provide an image of the potential applicability of liposomal carrier systems for nebulizer therapy. Regional lung drug deposition is patient-specific; therefore, deposition results might vary for different airway geometries. However, deposition measurement with realistic boundary conditions (airway geometry, breathing profile) brings a more realistic image of the drug delivery by the selected technology. Our results show how much data from cascade impactor testing or estimates from the fine fraction concept differ from those of a more realistic case.
Keywords: aerosol; deposition; inhalation; liposome; nebulizer; particle size; pulmonary drug delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures.Int J Pharm. 2007 Apr 4;334(1-2):62-70. doi: 10.1016/j.ijpharm.2006.10.022. Epub 2006 Oct 21. Int J Pharm. 2007. PMID: 17123757
-
Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers.Int J Pharm. 2021 Apr 1;598:120376. doi: 10.1016/j.ijpharm.2021.120376. Epub 2021 Feb 20. Int J Pharm. 2021. PMID: 33617949
-
Design principles of liquid nebulization devices currently in use.Respir Care. 2002 Nov;47(11):1257-75; discussion 1275-8. Respir Care. 2002. PMID: 12425742 Review.
-
Not all nebulizers are created equal: Considerations in choosing a nebulizer for aerosol delivery during mechanical ventilation.Expert Rev Respir Med. 2023 Feb;17(2):131-142. doi: 10.1080/17476348.2023.2183194. Epub 2023 Feb 27. Expert Rev Respir Med. 2023. PMID: 36803134 Review.
Cited by
-
Inhalable Nanotechnology-Based Drug Delivery Systems for the Treatment of Inflammatory Lung Diseases.Pharmaceutics. 2025 Jul 9;17(7):893. doi: 10.3390/pharmaceutics17070893. Pharmaceutics. 2025. PMID: 40733101 Free PMC article. Review.
-
Inhalational Drug Devices: Revisiting the Linchpin of Asthma Management.J Pers Med. 2024 Aug 16;14(8):867. doi: 10.3390/jpm14080867. J Pers Med. 2024. PMID: 39202058 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

