Starvation-resistant cavefish reveal conserved mechanisms of starvation-induced hepatic lipotoxicity
- PMID: 38467419
- PMCID: PMC10927358
- DOI: 10.26508/lsa.202302458
Starvation-resistant cavefish reveal conserved mechanisms of starvation-induced hepatic lipotoxicity
Abstract
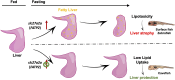
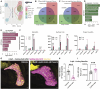
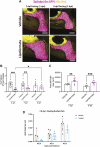
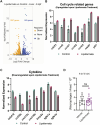
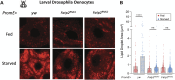
Starvation causes the accumulation of lipid droplets in the liver, a somewhat counterintuitive phenomenon that is nevertheless conserved from flies to humans. Much like fatty liver resulting from overfeeding, hepatic lipid accumulation (steatosis) during undernourishment can lead to lipotoxicity and atrophy of the liver. Here, we found that although surface populations of Astyanax mexicanus undergo this evolutionarily conserved response to starvation, the starvation-resistant cavefish larvae of the same species do not display an accumulation of lipid droplets upon starvation. Moreover, cavefish are resistant to liver atrophy during starvation, providing a unique system to explore strategies for liver protection. Using comparative transcriptomics between zebrafish, surface fish, and cavefish, we identified the fatty acid transporter slc27a2a/fatp2 to be correlated with the development of fatty liver. Pharmacological inhibition of slc27a2a in zebrafish rescues steatosis and atrophy of the liver upon starvation. Furthermore, down-regulation of FATP2 in Drosophila larvae inhibits the development of starvation-induced steatosis, suggesting the evolutionarily conserved importance of the gene in regulating fatty liver upon nutrition deprivation. Overall, our study identifies a conserved, druggable target to protect the liver from atrophy during starvation.
© 2024 Pozo-Morales et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Update of
-
Starvation resistant cavefish reveal conserved mechanisms of starvation-induced hepatic lipotoxicity.bioRxiv [Preprint]. 2024 Jan 11:2024.01.10.574986. doi: 10.1101/2024.01.10.574986. bioRxiv. 2024. Update in: Life Sci Alliance. 2024 Mar 11;7(5):e202302458. doi: 10.26508/lsa.202302458. PMID: 38260657 Free PMC article. Updated. Preprint.
Similar articles
-
Starvation resistant cavefish reveal conserved mechanisms of starvation-induced hepatic lipotoxicity.bioRxiv [Preprint]. 2024 Jan 11:2024.01.10.574986. doi: 10.1101/2024.01.10.574986. bioRxiv. 2024. Update in: Life Sci Alliance. 2024 Mar 11;7(5):e202302458. doi: 10.26508/lsa.202302458. PMID: 38260657 Free PMC article. Updated. Preprint.
-
A Model Construction of Starvation Induces Hepatic Steatosis and Transcriptome Analysis in Zebrafish Larvae.Biology (Basel). 2021 Jan 27;10(2):92. doi: 10.3390/biology10020092. Biology (Basel). 2021. PMID: 33513687 Free PMC article.
-
Early adipogenesis contributes to excess fat accumulation in cave populations of Astyanax mexicanus.Dev Biol. 2018 Sep 15;441(2):297-304. doi: 10.1016/j.ydbio.2018.06.003. Epub 2018 Jun 5. Dev Biol. 2018. PMID: 29883659
-
Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution.J Exp Biol. 2020 Feb 7;223(Pt Suppl 1):jeb208835. doi: 10.1242/jeb.208835. J Exp Biol. 2020. PMID: 32034044 Review.
-
Cavefish as a model system in evolutionary developmental biology.Dev Biol. 2001 Mar 1;231(1):1-12. doi: 10.1006/dbio.2000.0121. Dev Biol. 2001. PMID: 11180948 Review.
Cited by
-
Postprandial sleep in short-sleeping Mexican cavefish.bioRxiv [Preprint]. 2024 Jul 5:2024.07.03.602003. doi: 10.1101/2024.07.03.602003. bioRxiv. 2024. Update in: J Exp Zool A Ecol Integr Physiol. 2024 Dec;341(10):1084-1096. doi: 10.1002/jez.2880. PMID: 39005273 Free PMC article. Updated. Preprint.
-
Cholangiocytes contribute to hepatocyte regeneration after partial liver injury during growth spurt in zebrafish.Nat Commun. 2025 Jun 6;16(1):5260. doi: 10.1038/s41467-025-60334-y. Nat Commun. 2025. PMID: 40480975 Free PMC article.
-
Reproductive adaptation of Astyanax mexicanus under nutrient limitation.Dev Biol. 2025 Jul;523:82-98. doi: 10.1016/j.ydbio.2025.04.006. Epub 2025 Apr 11. Dev Biol. 2025. PMID: 40222642
-
Optimized methods to image hepatic lipid droplets in zebrafish larvae.Dis Model Mech. 2024 Nov 1;17(11):dmm050786. doi: 10.1242/dmm.050786. Epub 2024 Nov 25. Dis Model Mech. 2024. PMID: 39373223 Free PMC article.
-
Postprandial Sleep in Short-Sleeping Mexican Cavefish.J Exp Zool A Ecol Integr Physiol. 2024 Dec;341(10):1084-1096. doi: 10.1002/jez.2880. Epub 2024 Nov 13. J Exp Zool A Ecol Integr Physiol. 2024. PMID: 39539086 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
