Pharmacogenetic Influence on Stereoselective Steady-State Disposition of Bupropion
- PMID: 38467432
- PMCID: PMC11023817
- DOI: 10.1124/dmd.124.001697
Pharmacogenetic Influence on Stereoselective Steady-State Disposition of Bupropion
Abstract
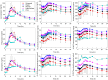
Bupropion is used for treating depression, obesity, and seasonal affective disorder, and for smoking cessation. Bupropion is commonly prescribed, but has complex pharmacokinetics and interindividual variability in metabolism and bioactivation may influence therapeutic response, tolerability, and safety. Bupropion is extensively and stereoselectively metabolized, the metabolites are pharmacologically active, and allelic variation in cytochrome P450 (CYP) 2B6 affects clinical hydroxylation of single-dose bupropion. Genetic effects on stereoselective disposition of steady-state bupropion are not known. In this preplanned secondary analysis of a prospective, randomized, double-blinded, crossover study which compared brand and generic bupropion XL 300 mg drug products, we measured steady-state enantiomeric plasma and urine parent bupropion and primary and secondary metabolite concentrations. This investigation evaluated the influence of genetic polymorphisms in CYP2B6, CYP2C19, and P450 oxidoreductase on the disposition of Valeant Pharmaceuticals Wellbutrin brand bupropion in 67 participants with major depressive disorder. We found that hydroxylation of both bupropion enantiomers was lower in carriers of the CYP2B6*6 allele and in carriers of the CYP2B6 516G>T variant, with correspondingly greater bupropion and lesser hydroxybupropion plasma concentrations. Hydroxylation was 25-50% lower in CYP2B6*6 carriers and one-third to one-half less in 516T carriers. Hydroxylation of the bupropion enantiomers was comparably affected by CYP2B6 variants. CYP2C19 polymorphisms did not influence bupropion plasma concentrations or hydroxybupropion formation but did influence the minor pathway of 4'-hydroxylation of bupropion and primary metabolites. P450 oxidoreductase variants did not influence bupropion disposition. Results show that CYP2B6 genetic variants affect steady-state metabolism and bioactivation of Valeant brand bupropion, which may influence therapeutic outcomes. SIGNIFICANCE STATEMENT: Bupropion, used for depression, obesity, and smoking cessation, undergoes metabolic bioactivation, with incompletely elucidated interindividual variability. We evaluated cytochrome P450 (CYP) 2B6, CYP2C19 and P450 oxidoreductase genetic variants and steady-state bupropion and metabolite enantiomers disposition. Both enantiomers hydroxylation was lower in CYP2B6*6 and CYP2B6 516G>T carriers, with greater bupropion and lesser hydroxybupropion plasma concentrations. CYP2C19 polymorphisms did not affect bupropion or hydroxybupropion but did influence minor 4'-hydroxylation of bupropion and primary metabolites. CYP2B6 variants affect steady-state bupropion bioactivation, which may influence therapeutic outcomes.
Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





Similar articles
-
Stereoselective Bupropion Hydroxylation by Cytochrome P450 CYP2B6 and Cytochrome P450 Oxidoreductase Genetic Variants.Drug Metab Dispos. 2020 Jun;48(6):438-445. doi: 10.1124/dmd.119.090407. Epub 2020 Apr 1. Drug Metab Dispos. 2020. PMID: 32238417 Free PMC article.
-
Common Polymorphisms of CYP2B6 Influence Stereoselective Bupropion Disposition.Clin Pharmacol Ther. 2019 Jan;105(1):142-152. doi: 10.1002/cpt.1116. Epub 2018 Aug 9. Clin Pharmacol Ther. 2019. PMID: 29756345 Free PMC article. Clinical Trial.
-
The P450 oxidoreductase (POR) rs2868177 and cytochrome P450 (CYP) 2B6*6 polymorphisms contribute to the interindividual variability in human CYP2B6 activity.Eur J Clin Pharmacol. 2016 Oct;72(10):1205-1213. doi: 10.1007/s00228-016-2095-0. Epub 2016 Jul 20. Eur J Clin Pharmacol. 2016. PMID: 27439448 Clinical Trial.
-
Role of CYP2B6 pharmacogenomics in bupropion-mediated smoking cessation.J Clin Pharm Ther. 2019 Apr;44(2):174-179. doi: 10.1111/jcpt.12783. Epub 2018 Dec 21. J Clin Pharm Ther. 2019. PMID: 30578565 Review.
-
Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations.Clin Ther. 2005 Nov;27(11):1685-95. doi: 10.1016/j.clinthera.2005.11.011. Clin Ther. 2005. PMID: 16368442 Review.
References
-
- Bousman CAStevenson JMRamsey LBSangkuhl KHicks JKStrawn JRSingh ABRuaño GMueller DJTsermpini EE, et al. (2023) Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin Pharmacol Ther 114:51–68. - PMC - PubMed
-
- Chen Y, Liu HF, Liu L, Nguyen K, Jones EB, Fretland AJ (2010) The in vitro metabolism of bupropion revisited: concentration dependent involvement of cytochrome P450 2C19. Xenobiotica 40:536–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
