Cervical spinal cord hemisection impacts sigh and the respiratory reset in male rats
- PMID: 38467570
- PMCID: PMC10927604
- DOI: 10.14814/phy2.15973
Cervical spinal cord hemisection impacts sigh and the respiratory reset in male rats
Abstract
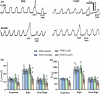
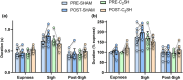
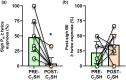
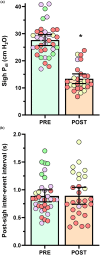
Cervical spinal cord injury impacts ventilatory and non-ventilatory functions of the diaphragm muscle (DIAm) and contributes to clinical morbidity and mortality in the afflicted population. Periodically, integrated brainstem neural circuit activity drives the DIAm to generate a markedly augmented effort or sigh-which plays an important role in preventing atelectasis and thus maintaining lung function. Across species, the general pattern of DIAm efforts during a normal sigh is variable in amplitude and the extent of post-sigh "apnea" (i.e., the post-sigh inter-breath interval). This post-sigh inter-breath interval acts as a respiratory reset, following the interruption of regular respiratory rhythm by sigh. We examined the impact of upper cervical (C2 ) spinal cord hemisection (C2 SH) on the transdiaphragmatic pressure (Pdi ) generated during sighs and the post-sigh respiratory reset in rats. Sighs were identified in Pdi traces by their characteristic biphasic pattern. We found that C2 SH results in a reduction of Pdi during both eupnea and sighs, and a decrease in the immediate post-sigh breath interval. These results are consistent with partial removal of descending excitatory synaptic inputs to phrenic motor neurons that results from C2 SH. Following cervical spinal cord injury, a reduction in the amplitude of Pdi during sighs may compromise the maintenance of normal lung function.
Keywords: diaphragm; respiration; spinal cord; transdiaphragmatic pressure; ventilation.
© 2024 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None of the authors has any conflicts of interest, real nor perceived, to disclose.
Figures

Similar articles
-
Novel regenerative drug, SPG302 promotes functional recovery of diaphragm muscle activity after cervical spinal cord injury.J Physiol. 2023 Jun;601(12):2513-2532. doi: 10.1113/JP284004. Epub 2023 Mar 7. J Physiol. 2023. PMID: 36815402 Free PMC article.
-
BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury.J Neurophysiol. 2017 Feb 1;117(2):537-544. doi: 10.1152/jn.00654.2016. Epub 2016 Nov 9. J Neurophysiol. 2017. PMID: 27832605 Free PMC article.
-
Acute intrathecal BDNF enhances functional recovery after cervical spinal cord injury in rats.J Neurophysiol. 2021 Jun 1;125(6):2158-2165. doi: 10.1152/jn.00146.2021. Epub 2021 May 5. J Neurophysiol. 2021. PMID: 33949892 Free PMC article.
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
-
Serotonergic innervation of respiratory motor nuclei after cervical spinal injury: Impact of intermittent hypoxia.Exp Neurol. 2021 Apr;338:113609. doi: 10.1016/j.expneurol.2021.113609. Epub 2021 Jan 15. Exp Neurol. 2021. PMID: 33460645 Free PMC article. Review.
Cited by
-
Identification of eupneic breathing using machine learning.J Neurophysiol. 2024 Sep 1;132(3):678-684. doi: 10.1152/jn.00230.2024. Epub 2024 Jul 25. J Neurophysiol. 2024. PMID: 39052237
-
Cyclic sighing in the clinic waiting room may decrease pain: results from a pilot randomized controlled trial.J Behav Med. 2025 Apr;48(2):385-393. doi: 10.1007/s10865-024-00548-5. Epub 2025 Feb 4. J Behav Med. 2025. PMID: 39904867 Clinical Trial.
-
Dendritic alterations precede age-related dysphagia and nucleus ambiguus motor neuron death.J Physiol. 2025 Mar;603(5):1299-1321. doi: 10.1113/JP287457. Epub 2025 Jan 27. J Physiol. 2025. PMID: 39868939 Free PMC article.
References
-
- Bell, H. J. , Azubike, E. , & Haouzi, P. (2011). The “other” respiratory effect of opioids: Suppression of spontaneous augmented (“sigh”) breaths. Journal of Applied Physiology, 111, 1296–1303. - PubMed
-
- Bell, H. J. , Ferguson, C. , Kehoe, V. , & Haouzi, P. (2009). Hypocapnia increases the prevalence of hypoxia‐induced augmented breaths. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 296, R334–R344. - PubMed
-
- Bell, H. J. , & Haouzi, P. (2010). The hypoxia‐induced facilitation of augmented breaths is suppressed by the common effect of carbonic anhydrase inhibition. Respiratory Physiology & Neurobiology, 171, 201–211. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

