Silencing CA1 pyramidal cells output reveals the role of feedback inhibition in hippocampal oscillations
- PMID: 38467602
- PMCID: PMC10928166
- DOI: 10.1038/s41467-024-46478-3
Silencing CA1 pyramidal cells output reveals the role of feedback inhibition in hippocampal oscillations
Abstract
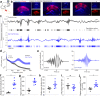
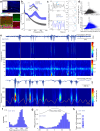
The precise temporal coordination of neural activity is crucial for brain function. In the hippocampus, this precision is reflected in the oscillatory rhythms observed in CA1. While it is known that a balance between excitatory and inhibitory activity is necessary to generate and maintain these oscillations, the differential contribution of feedforward and feedback inhibition remains ambiguous. Here we use conditional genetics to chronically silence CA1 pyramidal cell transmission, ablating the ability of these neurons to recruit feedback inhibition in the local circuit, while recording physiological activity in mice. We find that this intervention leads to local pathophysiological events, with ripple amplitude and intrinsic frequency becoming significantly larger and spatially triggered local population spikes locked to the trough of the theta oscillation appearing during movement. These phenotypes demonstrate that feedback inhibition is crucial in maintaining local sparsity of activation and reveal the key role of lateral inhibition in CA1 in shaping circuit function.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Battaglia FP, Benchenane K, Sirota A, Pennartz CMA, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn. Sci. 2011;15:310–318. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

