A first-in-class TIMM44 blocker inhibits bladder cancer cell growth
- PMID: 38467612
- PMCID: PMC10928220
- DOI: 10.1038/s41419-024-06585-x
A first-in-class TIMM44 blocker inhibits bladder cancer cell growth
Abstract
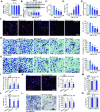
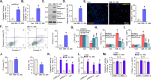
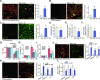
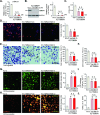
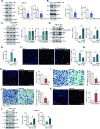
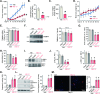
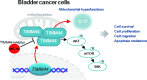
Mitochondria play a multifaceted role in supporting bladder cancer progression. Translocase of inner mitochondrial membrane 44 (TIMM44) is essential for maintaining function and integrity of mitochondria. We here tested the potential effect of MB-10 (MitoBloCK-10), a first-in-class TIMM44 blocker, against bladder cancer cells. TIMM44 mRNA and protein expression is significantly elevated in both human bladder cancer tissues and cells. In both patient-derived primary bladder cancer cells and immortalized (T24) cell line, MB-10 exerted potent anti-cancer activity and inhibited cell viability, proliferation and motility. The TIMM44 blocker induced apoptosis and cell cycle arrest in bladder cancer cells, but failed to provoke cytotoxicity in primary bladder epithelial cells. MB-10 disrupted mitochondrial functions in bladder cancer cells, causing mitochondrial depolarization, oxidative stress and ATP reduction. Whereas exogenously-added ATP and the antioxidant N-Acetyl Cysteine mitigated MB-10-induced cytotoxicity of bladder cancer cells. Genetic depletion of TIMM44 through CRISPR-Cas9 method also induced robust anti-bladder cancer cell activity and MB-10 had no effect in TIMM44-depleted cancer cells. Contrarily, ectopic overexpression of TIMM44 using a lentiviral construct augmented proliferation and motility of primary bladder cancer cells. TIMM44 is important for Akt-mammalian target of rapamycin (mTOR) activation. In primary bladder cancer cells, Akt-S6K1 phosphorylation was decreased by MB-10 treatment or TIMM44 depletion, but enhanced after ectopic TIMM44 overexpression. In vivo, intraperitoneal injection of MB-10 impeded bladder cancer xenograft growth in nude mice. Oxidative stress, ATP reduction, Akt-S6K1 inhibition and apoptosis were detected in MB-10-treated xenograft tissues. Moreover, genetic depletion of TIMM44 also arrested bladder cancer xenograft growth in nude mice, leading to oxidative stress, ATP reduction and Akt-S6K1 inhibition in xenograft tissues. Together, targeting overexpressed TIMM44 by MB-10 significantly inhibits bladder cancer cell growth in vitro and in vivo.
© 2024. The Author(s).
Conflict of interest statement
In this study, all authors affirm that the research was carried out without any commercial or financial associations that might raise concerns about potential conflicts of interest. There are no reported conflicts of interest among the authors.
Figures



Similar articles
-
Targeting POLRMT by IMT1 inhibits colorectal cancer cell growth.Cell Death Dis. 2024 Sep 3;15(9):643. doi: 10.1038/s41419-024-07023-8. Cell Death Dis. 2024. PMID: 39227564 Free PMC article.
-
The mitochondrial protein TIMM44 is required for angiogenesis in vitro and in vivo.Cell Death Dis. 2023 May 5;14(5):307. doi: 10.1038/s41419-023-05826-9. Cell Death Dis. 2023. PMID: 37147302 Free PMC article.
-
TIMM44 is a potential therapeutic target of human glioma.Theranostics. 2022 Oct 31;12(17):7586-7602. doi: 10.7150/thno.78616. eCollection 2022. Theranostics. 2022. PMID: 36438483 Free PMC article.
-
Targeting the mitochondrial protein YME1L to inhibit osteosarcoma cell growth in vitro and in vivo.Cell Death Dis. 2024 May 20;15(5):346. doi: 10.1038/s41419-024-06722-6. Cell Death Dis. 2024. PMID: 38769124 Free PMC article.
-
Targeting POLRMT by a first-in-class inhibitor IMT1 inhibits osteosarcoma cell growth in vitro and in vivo.Cell Death Dis. 2024 Jan 16;15(1):57. doi: 10.1038/s41419-024-06444-9. Cell Death Dis. 2024. PMID: 38228583 Free PMC article.
Cited by
-
Identification of mitochondrial carrier homolog 2 as an important therapeutic target of castration-resistant prostate cancer.Cell Death Dis. 2025 Feb 5;16(1):70. doi: 10.1038/s41419-025-07406-5. Cell Death Dis. 2025. PMID: 39910035 Free PMC article.
-
Ferroptosis-related lncRNA AL136084.3 is associated with NUPR1 in bladder cancer.Discov Oncol. 2024 Nov 30;15(1):730. doi: 10.1007/s12672-024-01564-2. Discov Oncol. 2024. PMID: 39613992 Free PMC article.
-
The expression and functional role of proline-rich 15 in non-small cell lung cancer.Cell Death Dis. 2025 Feb 10;16(1):83. doi: 10.1038/s41419-025-07373-x. Cell Death Dis. 2025. PMID: 39929816 Free PMC article.
-
Mitochondrial metabolism and cancer therapeutic innovation.Signal Transduct Target Ther. 2025 Aug 4;10(1):245. doi: 10.1038/s41392-025-02311-x. Signal Transduct Target Ther. 2025. PMID: 40754534 Free PMC article. Review.
-
Targeting POLRMT by IMT1 inhibits colorectal cancer cell growth.Cell Death Dis. 2024 Sep 3;15(9):643. doi: 10.1038/s41419-024-07023-8. Cell Death Dis. 2024. PMID: 39227564 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

