Protein expression of nucleolar protein 12 in the retina and its implication in protection of retina from UV irradiation damage
- PMID: 38467618
- PMCID: PMC10928217
- DOI: 10.1038/s41420-024-01902-x
Protein expression of nucleolar protein 12 in the retina and its implication in protection of retina from UV irradiation damage
Abstract
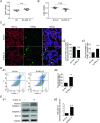
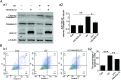
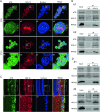
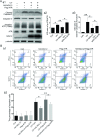
Nucleolar protein 12 (NOL12), one of the nucleolar proteins which are primarily expressed in the nucleolus and play key roles in RNA metabolism, cell proliferation, cell cycle, and cell survival, is widely expressed in various species and multiple organs. Although it has been reported that the mRNA of Drosophila NOL12 homolog viriato is expressed in the eyes of Drosophila, the protein expression of NOL12 in mammalian eyes remains to be elucidated. In this study, we showed through immunohistochemistry that NOL12 was present in the rat retina, with predominant distribution in the cytoplasm of the retinal neuronal cells. In the human retinoblastoma cell line WERI-Rb1, we found that altering NOL12 expression led to a change in WERI-Rb1 cell viability. Knocking down NOL12 expression decreased cell viability. In contrast, overexpressing NOL12 increased cell viability. Furthermore, increasing NOL12 expression inhibited ultraviolet (UV)-induced apoptosis. These findings demonstrated that NOL12 may play an important protective role in retinal cells. In the WERI-Rb1 cells exposed to UV irradiation, we detected that NOL12 was degraded, but this degradation could be attenuated by a pan-Caspase inhibitor. Notably, the inhibitory effect of NOL12 against UV-induced apoptosis could be restrained by increasing the expression of ATR serine/threonine kinase (ATR), a kinase that, when activated by severe DNA damage, can result in apoptosis. We also found that upregulating NOL12 inhibited the activation of ATR caused by UV irradiation. Additionally, inhibiting ATR activity reduced apoptosis resulting from both silencing NOL12 expression and UV exposure. Thus, NOL12 may protect against UV irradiation-induced retinal damage by inhibiting ATR activity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The Drosophila Nol12 homologue viriato is a dMyc target that regulates nucleolar architecture and is required for dMyc-stimulated cell growth.Development. 2011 Jan;138(2):349-57. doi: 10.1242/dev.054411. Development. 2011. PMID: 21177347
-
NOL12 Repression Induces Nucleolar Stress-Driven Cellular Senescence and Is Associated with Normative Aging.Mol Cell Biol. 2019 May 28;39(12):e00099-19. doi: 10.1128/MCB.00099-19. Print 2019 Jun 15. Mol Cell Biol. 2019. PMID: 30988155 Free PMC article.
-
Nol12 is a multifunctional RNA binding protein at the nexus of RNA and DNA metabolism.Nucleic Acids Res. 2017 Dec 1;45(21):12509-12528. doi: 10.1093/nar/gkx963. Nucleic Acids Res. 2017. PMID: 29069457 Free PMC article.
-
Targeting protective autophagy exacerbates UV-triggered apoptotic cell death.Int J Mol Sci. 2012;13(1):1209-1224. doi: 10.3390/ijms13011209. Epub 2012 Jan 20. Int J Mol Sci. 2012. PMID: 22312313 Free PMC article.
-
Differential induction of proto-oncogene expression and cell death in ocular tissues following ultraviolet irradiation of the rat eye.Br J Ophthalmol. 1999 Feb;83(2):225-30. doi: 10.1136/bjo.83.2.225. Br J Ophthalmol. 1999. PMID: 10396203 Free PMC article.
Cited by
-
Prevalence and associated factors of vitreoretinal interface disorders using multicolour OCT among Chinese population in Fujian eye study.Sci Rep. 2025 Aug 1;15(1):28174. doi: 10.1038/s41598-025-12717-w. Sci Rep. 2025. PMID: 40751068 Free PMC article.
References
Grants and funding
- 81971199/National Natural Science Foundation of China (National Science Foundation of China)
- 31971114/National Natural Science Foundation of China (National Science Foundation of China)
- 81971199/National Natural Science Foundation of China (National Science Foundation of China)
- 31971114/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Miscellaneous

