Delivery of a BET protein degrader via a CEACAM6-targeted antibody-drug conjugate inhibits tumour growth in pancreatic cancer models
- PMID: 38467634
- PMCID: PMC10928091
- DOI: 10.1038/s41467-024-46167-1
Delivery of a BET protein degrader via a CEACAM6-targeted antibody-drug conjugate inhibits tumour growth in pancreatic cancer models
Abstract
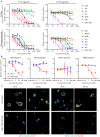
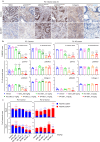
Pancreatic ductal adenocarcinoma (PDAC) has the worst prognosis of all cancers. To improve PDAC therapy, we establish screening systems based on organoid and co-culture technologies and find a payload of antibody-drug conjugate (ADC), a bromodomain and extra-terminal (BET) protein degrader named EBET. We select CEACAM6/CD66c as an ADC target and developed an antibody, #84.7, with minimal reactivity to CEACAM6-expressing normal cells. EBET-conjugated #84.7 (84-EBET) has lethal effects on various PDAC organoids and bystander efficacy on CEACAM6-negative PDAC cells and cancer-associated fibroblasts. In mouse studies, a single injection of 84-EBET induces marked tumor regression in various PDAC-patient-derived xenografts, with a decrease in the inflammatory phenotype of stromal cells and without significant body weight loss. Combination with standard chemotherapy or PD-1 antibody induces more profound and sustained regression without toxicity enhancement. Our preclinical evidence demonstrates potential efficacy by delivering BET protein degrader to PDAC and its microenvironment via CEACAM6-targeted ADC.
© 2024. The Author(s).
Conflict of interest statement
All authors are employees of Eisai Co., Ltd. and this study was funded by Eisai Co., Ltd.
Figures




Similar articles
-
Broad-Spectrum Efficacy of CEACAM6-Targeted Antibody-Drug Conjugate with BET Protein Degrader in Colorectal, Lung, and Breast Cancer Mouse Models.Mol Cancer Ther. 2025 Mar 4;24(3):392-405. doi: 10.1158/1535-7163.MCT-24-0444. Mol Cancer Ther. 2025. PMID: 39812376 Free PMC article.
-
Preclinical evaluation of carcinoembryonic cell adhesion molecule (CEACAM) 6 as potential therapy target for pancreatic adenocarcinoma.J Pathol. 2009 Jul;218(3):380-90. doi: 10.1002/path.2545. J Pathol. 2009. PMID: 19334050
-
CT109-SN-38, a Novel Antibody-drug Conjugate with Dual Specificity for CEACAM5 and 6, Elicits Potent Killing of Pancreatic Cancer Cells.Curr Cancer Drug Targets. 2024;24(7):720-732. doi: 10.2174/0115680096260614231115192343. Curr Cancer Drug Targets. 2024. PMID: 38178674
-
Preclinical models of pancreatic ductal adenocarcinoma.Chin Clin Oncol. 2017 Jun;6(3):25. doi: 10.21037/cco.2017.06.15. Chin Clin Oncol. 2017. PMID: 28705002 Review.
-
Modeling pancreatic cancer in mice for experimental therapeutics.Biochim Biophys Acta Rev Cancer. 2021 Aug;1876(1):188554. doi: 10.1016/j.bbcan.2021.188554. Epub 2021 May 1. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33945847 Free PMC article. Review.
Cited by
-
Targeted Suppression of CEACAM6 via pHLIP-Delivered RNAs in Pancreatic Ductal Adenocarcinoma.Medicina (Kaunas). 2025 Mar 26;61(4):598. doi: 10.3390/medicina61040598. Medicina (Kaunas). 2025. PMID: 40282889 Free PMC article.
-
Harnessing Organoid Platforms for Nanoparticle Drug Development.Drug Des Devel Ther. 2025 Jul 18;19:6125-6143. doi: 10.2147/DDDT.S530999. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40698061 Free PMC article. Review.
-
Diagnostic value of CEACAM6 and HE4 in pleural fluid for malignant pleural effusion.Ann Med. 2025 Dec;57(1):2489748. doi: 10.1080/07853890.2025.2489748. Epub 2025 Apr 15. Ann Med. 2025. PMID: 40232301 Free PMC article.
-
Advancing pancreatic cancer research and therapeutics: the transformative role of organoid technology.Exp Mol Med. 2025 Feb;57(1):50-58. doi: 10.1038/s12276-024-01378-w. Epub 2025 Jan 16. Exp Mol Med. 2025. PMID: 39814914 Free PMC article. Review.
-
[CEACAM6 inhibits proliferation and migration of nasopharyngeal carcinoma cells by suppressing epithelial-mesenchymal transition].Nan Fang Yi Ke Da Xue Xue Bao. 2025 Mar 20;45(3):566-576. doi: 10.12122/j.issn.1673-4254.2025.03.14. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40159971 Free PMC article. Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

