YTHDF2 governs muscle size through a targeted modulation of proteostasis
- PMID: 38467649
- PMCID: PMC10928198
- DOI: 10.1038/s41467-024-46546-8
YTHDF2 governs muscle size through a targeted modulation of proteostasis
Abstract
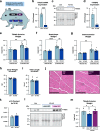
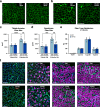
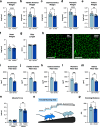
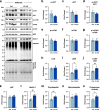
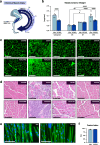
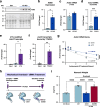
The regulation of proteostasis is fundamental for maintenance of muscle mass and function. Activation of the TGF-β pathway drives wasting and premature aging by favoring the proteasomal degradation of structural muscle proteins. Yet, how this critical post-translational mechanism is kept in check to preserve muscle health remains unclear. Here, we reveal the molecular link between the post-transcriptional regulation of m6A-modified mRNA and the modulation of SMAD-dependent TGF-β signaling. We show that the m6A-binding protein YTHDF2 is essential to determining postnatal muscle size. Indeed, muscle-specific genetic deletion of YTHDF2 impairs skeletal muscle growth and abrogates the response to hypertrophic stimuli. We report that YTHDF2 controls the mRNA stability of the ubiquitin ligase ASB2 with consequences on anti-growth gene program activation through SMAD3. Our study identifies a post-transcriptional to post-translational mechanism for the coordination of gene expression in muscle.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Runx2/Smad3 complex negatively regulates TGF-β-induced connective tissue growth factor gene expression in vascular smooth muscle cells.J Atheroscler Thromb. 2012;19(1):23-35. doi: 10.5551/jat.9753. Epub 2011 Oct 8. J Atheroscler Thromb. 2012. PMID: 21986102
-
YAP/TAZ regulates TGF-β/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts.Cell Commun Signal. 2018 Apr 25;16(1):18. doi: 10.1186/s12964-018-0232-3. Cell Commun Signal. 2018. PMID: 29695252 Free PMC article.
-
Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer metastasis.J Clin Invest. 2014 Feb;124(2):564-79. doi: 10.1172/JCI71104. Epub 2014 Jan 2. J Clin Invest. 2014. PMID: 24382352 Free PMC article.
-
KLF17 empowers TGF-β/Smad signaling by targeting Smad3-dependent pathway to suppress tumor growth and metastasis during cancer progression.Cell Death Dis. 2015 Mar 12;6(3):e1681. doi: 10.1038/cddis.2015.48. Cell Death Dis. 2015. PMID: 25766320 Free PMC article.
-
A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling.J Cell Biochem. 2007 May 1;101(1):9-33. doi: 10.1002/jcb.21255. J Cell Biochem. 2007. PMID: 17340614 Review.
Cited by
-
Ythdf2 facilitates precursor miR-378/miR-378-5p maturation to support myogenic differentiation.Cell Mol Life Sci. 2024 Nov 6;81(1):445. doi: 10.1007/s00018-024-05456-0. Cell Mol Life Sci. 2024. PMID: 39503763 Free PMC article.
-
At the Nexus Between Epigenetics and Senescence: The Effects of Senolytic (BI01) Administration on DNA Methylation Clock Age and the Methylome in Aged and Regenerated Skeletal Muscle.Aging Cell. 2025 Jul;24(7):e70068. doi: 10.1111/acel.70068. Epub 2025 Apr 21. Aging Cell. 2025. PMID: 40259517 Free PMC article.
-
Regulation of cardiac hypertrophy by RNA readers.RNA. 2025 Jun 16;31(7):871-873. doi: 10.1261/rna.080557.125. RNA. 2025. PMID: 40316421 Free PMC article. No abstract available.
-
Methylome-proteome integration after late-life voluntary exercise training reveals regulation and target information for improved skeletal muscle health.J Physiol. 2025 Jan;603(1):211-237. doi: 10.1113/JP286681. Epub 2024 Jul 26. J Physiol. 2025. PMID: 39058663 Free PMC article.
-
Endurance exercise remodels skeletal muscle by suppressing Ythdf1-mediated myostatin expression.Cell Death Dis. 2025 Feb 13;16(1):96. doi: 10.1038/s41419-025-07379-5. Cell Death Dis. 2025. PMID: 39948064 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

