Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
- PMID: 38467660
- PMCID: PMC10928171
- DOI: 10.1038/s41467-024-46550-y
Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
Abstract
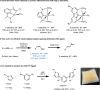
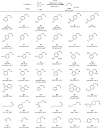
Stereodefined vinylboron compounds are important organic synthons. The synthesis of E-1-vinylboron compounds typically involves the addition of a B-H bond to terminal alkynes. The selective generation of the thermodynamically unfavorable Z-isomers remains challenging, necessitating improved methods. Here, such a proficient and cost-effective catalytic system is introduced, comprising a cobalt salt and a readily accessible air-stable CNC pincer ligand. This system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with excellent Z-selectivity. High turnover numbers (>1,600) and turnover frequencies (>132,000 h-1) are achieved at room temperature, and the reaction can be scaled up to 30 mmol smoothly. Kinetic studies reveal a formal second-order dependence on cobalt concentration. Mechanistic investigations indicate that the alkynes exhibit a higher affinity for the catalyst than the alkene products, resulting in exceptional Z-selective performance. Furthermore, a rare time-dependent stereoselectivity is observed, allowing for quantitative conversion of Z-vinylboronate esters to the E-isomers.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
-
- Yoshida H. Borylation of alkynes under base/coinage metal catalysis: some recent developments. ACS Catal. 2016;6:1799–1811. doi: 10.1021/acscatal.5b02973. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

