Disrupted sleep-wake regulation in the MCI-Park mouse model of Parkinson's disease
- PMID: 38467673
- PMCID: PMC10928107
- DOI: 10.1038/s41531-024-00670-w
Disrupted sleep-wake regulation in the MCI-Park mouse model of Parkinson's disease
Erratum in
-
Author Correction: Disrupted sleep-wake regulation in the MCI-Park mouse model of Parkinson's disease.NPJ Parkinsons Dis. 2024 Jul 15;10(1):131. doi: 10.1038/s41531-024-00746-7. NPJ Parkinsons Dis. 2024. PMID: 39009637 Free PMC article. No abstract available.
Abstract
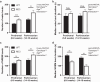
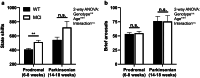
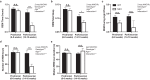
Disrupted sleep has a profound adverse impact on lives of Parkinson's disease (PD) patients and their caregivers. Sleep disturbances are exceedingly common in PD, with substantial heterogeneity in type, timing, and severity. Among the most common sleep-related symptoms reported by PD patients are insomnia, excessive daytime sleepiness, and sleep fragmentation, characterized by interruptions and decreased continuity of sleep. Alterations in brain wave activity, as measured on the electroencephalogram (EEG), also occur in PD, with changes in the pattern and relative contributions of different frequency bands of the EEG spectrum to overall EEG activity in different vigilance states consistently observed. The mechanisms underlying these PD-associated sleep-wake abnormalities are poorly understood, and they are ineffectively treated by conventional PD therapies. To help fill this gap in knowledge, a new progressive model of PD - the MCI-Park mouse - was studied. Near the transition to the parkinsonian state, these mice exhibited significantly altered sleep-wake regulation, including increased wakefulness, decreased non-rapid eye movement (NREM) sleep, increased sleep fragmentation, reduced rapid eye movement (REM) sleep, and altered EEG activity patterns. These sleep-wake abnormalities resemble those identified in PD patients. Thus, this model may help elucidate the circuit mechanisms underlying sleep disruption in PD and identify targets for novel therapeutic approaches.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

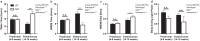
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

