Antifungal activity of Fe3O4@SiO2/Schiff-base/Cu(II) magnetic nanoparticles against pathogenic Candida species
- PMID: 38467729
- PMCID: PMC10928175
- DOI: 10.1038/s41598-024-56512-5
Antifungal activity of Fe3O4@SiO2/Schiff-base/Cu(II) magnetic nanoparticles against pathogenic Candida species
Abstract
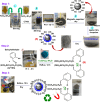
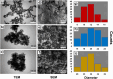
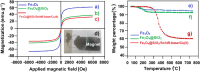
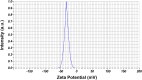
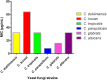
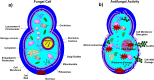
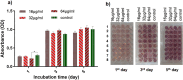
The antifungal efficacy and cytotoxicity of a novel nano-antifungal agent, the Fe3O4@SiO2/Schiff-base complex of Cu(II) magnetic nanoparticles (MNPs), have been assessed for targeting drug-resistant Candida species. Due to the rising issue of fungal infections, especially candidiasis, and resistance to traditional antifungals, there is an urgent need for new therapeutic strategies. Utilizing Schiff-base ligands known for their broad-spectrum antimicrobial activity, the Fe3O4@SiO2/Schiff-base/Cu(II) MNPs have been synthesized. The Fe3O4@SiO2/Schiff-base/Cu(II) MNPs was characterized by Fourier Transform-Infrared Spectroscopy (FT-IR), X-ray Diffraction (XRD), Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), Dynamic Light Scattering (DLS), Energy-dispersive X-ray (EDX), Vibrating Sample Magnetometer (VSM), and Thermogravimetric analysis (TGA), demonstrating successful synthesis. The antifungal potential was evaluated against six Candida species (C. dubliniensis, C. krusei, C. tropicalis, C. parapsilosis, C. glabrata, and C. albicans) using the broth microdilution method. The results indicated strong antifungal activity in the range of 8-64 μg/mL with the lowest MIC (8 μg/mL) observed against C. parapsilosis. The result showed the MIC of 32 μg/mL against C. albicans as the most common infection source. The antifungal mechanism is likely due to the disruption of the fungal cell wall and membrane, along with increased reactive oxygen species (ROS) generation leading to cell death. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay for cytotoxicity on mouse L929 fibroblastic cells suggested low toxicity and even enhanced cell proliferation at certain concentrations. This study demonstrates the promise of Fe3O4@SiO2/Schiff-base/Cu(II) MNPs as a potent antifungal agent with potential applications in the treatment of life-threatening fungal infections, healthcare-associated infections, and beyond.
Keywords: Anti-fungal property; Candida species; Cu(II) Nanoparticles; Cytotoxicity; Microdilution.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ayanwale AP, Estrada-Capetillo BL, Reyes-López SY. Evaluation of antifungal activity by mixed oxide metallic nanocomposite against Candida spp. Processes. 2021;9:773–787. doi: 10.3390/pr9050773. - DOI
-
- Akturk A, Güler FK, Taygun ME, Goller G, Küçükbayrak S. Synthesis and antifungal activity of soluble starch and sodium alginate capped copper nanoparticles. Mater. Res. Express. 2019;6:1250–1263. doi: 10.1088/2053-1591/ab677e. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

