The 'communicatome' of pregnancy: spotlight on cellular and extravesicular chimerism
- PMID: 38467841
- PMCID: PMC11018796
- DOI: 10.1038/s44321-024-00045-x
The 'communicatome' of pregnancy: spotlight on cellular and extravesicular chimerism
Abstract
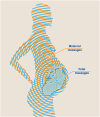
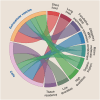
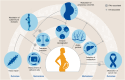
Communication via biological mediators between mother and fetus are key to reproductive success and offspring's future health. The repertoire of mediators coding signals between mother and fetus is broad and includes soluble factors, membrane-bound particles and immune as well as non-immune cells. Based on the emergence of technological advancements over the last years, considerable progress has been made toward deciphering the "communicatome" between fetus and mother during pregnancy and even after birth. In this context, pregnancy-associated chimerism has sparked the attention among immunologists, since chimeric cells-although low in number-are maintained in the allogeneic host (mother or fetus) for years after birth. Other non-cellular structures of chimerism, e.g. extracellular vesicles (EVs), are increasingly recognized as modulators of pregnancy outcome and offspring's health. We here discuss the origin, distribution and function of pregnancy-acquired microchimerism and chimeric EVs in mother and offspring. We also highlight the pioneering concept of maternal microchimeric cell-derived EVs in offspring. Such insights expand the understanding of pregnancy-associated health or disease risks in mother and offspring.
Keywords: Extracellular Vesicles; Feto-maternal Communication; Microchimerism; Pregnancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Albrecht M, Pagenkemper M, Wiessner C, Spohn M, Lütgehetmann M, Jacobsen H, Gabriel G, Zazara DE, Haertel C, Hecher K, et al. Infant immunity against viral infections is advanced by the placenta-dependent vertical transfer of maternal antibodies. Vaccine. 2022;40:1563–1571. doi: 10.1016/j.vaccine.2020.12.049. - DOI - PubMed
-
- Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee TH. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

