Group 3 innate lymphoid cells in intestinal health and disease
- PMID: 38467885
- PMCID: PMC11144103
- DOI: 10.1038/s41575-024-00906-3
Group 3 innate lymphoid cells in intestinal health and disease
Abstract
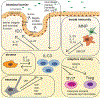
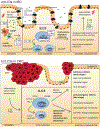
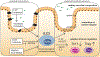
The gastrointestinal tract is an immunologically rich organ, containing complex cell networks and dense lymphoid structures that safeguard this large absorptive barrier from pathogens, contribute to tissue physiology and support mucosal healing. Simultaneously, the immune system must remain tolerant to innocuous dietary antigens and trillions of normally beneficial microorganisms colonizing the intestine. Indeed, a dysfunctional immune response in the intestine underlies the pathogenesis of numerous local and systemic diseases, including inflammatory bowel disease, food allergy, chronic enteric infections or cancers. Here, we discuss group 3 innate lymphoid cells (ILC3s), which have emerged as orchestrators of tissue physiology, immunity, inflammation, tolerance and malignancy in the gastrointestinal tract. ILC3s are abundant in the developing and healthy intestine but their numbers or function are altered during chronic disease and cancer. The latest studies provide new insights into the mechanisms by which ILC3s fundamentally shape intestinal homeostasis or disease pathophysiology, and often this functional dichotomy depends on context and complex interactions with other cell types or microorganisms. Finally, we consider how this knowledge could be harnessed to improve current treatments or provoke new opportunities for therapeutic intervention to promote gut health.
© 2024. Springer Nature Limited.
Figures

References
-
- Spits H. et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol 13, 145–149 (2013). - PubMed
-
- Vivier E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018). - PubMed
-
- Satoh-Takayama N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

