Single-molecule magnetic tweezers to probe the equilibrium dynamics of individual proteins at physiologically relevant forces and timescales
- PMID: 38467905
- PMCID: PMC7616092
- DOI: 10.1038/s41596-024-00965-5
Single-molecule magnetic tweezers to probe the equilibrium dynamics of individual proteins at physiologically relevant forces and timescales
Abstract
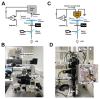
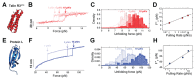
The reversible unfolding and refolding of proteins is a regulatory mechanism of tissue elasticity and signalling used by cells to sense and adapt to extracellular and intracellular mechanical forces. However, most of these proteins exhibit low mechanical stability, posing technical challenges to the characterization of their conformational dynamics under force. Here, we detail step-by-step instructions for conducting single-protein nanomechanical experiments using ultra-stable magnetic tweezers, which enable the measurement of the equilibrium conformational dynamics of single proteins under physiologically relevant low forces applied over biologically relevant timescales. We report the basic principles determining the functioning of the magnetic tweezer instrument, review the protein design strategy and the fluid chamber preparation and detail the procedure to acquire and analyze the unfolding and refolding trajectories of individual proteins under force. This technique adds to the toolbox of single-molecule nanomechanical techniques and will be of particular interest to those interested in proteins involved in mechanosensing and mechanotransduction. The procedure takes 4 d to complete, plus an additional 6 d for protein cloning and production, requiring basic expertise in molecular biology, surface chemistry and data analysis.
© 2024. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures











Similar articles
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Temperature-dependent funnel-like DNA folding landscapes.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf698. doi: 10.1093/nar/gkaf698. Nucleic Acids Res. 2025. PMID: 40682825 Free PMC article.
-
Proteomic Investigation of Immune Checkpoints and Some of Their Inhibitors.Int J Mol Sci. 2024 Aug 27;25(17):9276. doi: 10.3390/ijms25179276. Int J Mol Sci. 2024. PMID: 39273224 Free PMC article. Review.
-
Single-molecule magnetic tweezers to unravel protein folding dynamics under force.Biophys Rev. 2025 Feb 8;17(1):25-44. doi: 10.1007/s12551-025-01274-1. eCollection 2025 Feb. Biophys Rev. 2025. PMID: 40060007 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

