The TAM, a Translocation and Assembly Module for protein assembly and potential conduit for phospholipid transfer
- PMID: 38467907
- PMCID: PMC11014939
- DOI: 10.1038/s44319-024-00111-y
The TAM, a Translocation and Assembly Module for protein assembly and potential conduit for phospholipid transfer
Abstract
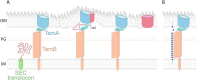
The assembly of β-barrel proteins into the bacterial outer membrane is an essential process enabling the colonization of new environmental niches. The TAM was discovered as a module of the β-barrel protein assembly machinery; it is a heterodimeric complex composed of an outer membrane protein (TamA) bound to an inner membrane protein (TamB). The TAM spans the periplasm, providing a scaffold through the peptidoglycan layer and catalyzing the translocation and assembly of β-barrel proteins into the outer membrane. Recently, studies on another membrane protein (YhdP) have suggested that TamB might play a role in phospholipid transport to the outer membrane. Here we review and re-evaluate the literature covering the experimental studies on the TAM over the past decade, to reconcile what appear to be conflicting claims on the function of the TAM.
Keywords: BAM Complex; Beta-barrel Proteins; Lipid Transport; Outer Membrane Biogenesis; TamB.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bamert RS, Lundquist K, Hwang H, Webb CT, Shiota T, Stubenrauch CJ, Belousoff MJ, Goode RJA, Schittenhelm RB, Zimmerman R, et al. Structural basis for substrate selection by the translocation and assembly module of the beta-barrel assembly machinery. Mol Microbiol. 2017;106:142–156. doi: 10.1111/mmi.13757. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

