Real-World Effectiveness of Upadacitinib for Treatment of Rheumatoid Arthritis in Canadian Patients: Interim Results from the Prospective Observational CLOSE-UP Study
- PMID: 38467912
- PMCID: PMC11111641
- DOI: 10.1007/s40744-024-00651-8
Real-World Effectiveness of Upadacitinib for Treatment of Rheumatoid Arthritis in Canadian Patients: Interim Results from the Prospective Observational CLOSE-UP Study
Abstract
Introduction: Upadacitinib (UPA), a selective, reversible, oral Janus kinase (JAK)-1 inhibitor, was approved in 2019 in Canada for the treatment of adults with moderately to severely active rheumatoid arthritis (RA). This phase 4 prospective study aimed to characterise the effectiveness of UPA in the real-world population of patients with RA.
Methods: Adults with RA who initiated treatment with once daily UPA (15 mg) and enrolled in the Canadian Real-Life post-marketing Observational Study assessing the Effectiveness of UPadacitinib for treating rheumatoid arthritis (CLOSE-UP) and who completed a 6-month assessment as of 28 February 2023 were included. The primary endpoint of the CLOSE-UP study is the proportion of patients achieving a Disease Activity Score-28 Joint Count C-reactive protein (DAS28-CRP) < 2.6 at 6 months. Data was collected at routine visits. Data analysed and summarised descriptively for the overall interim population and for subgroups based on prior therapy included remission or low disease activity, patient-reported outcomes (PROs), and adverse events.
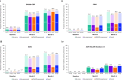
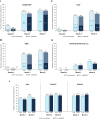
Results: A total of 392 patients were included in the interim analysis. Overall, 63.5% (191/301) of patients achieved a DAS28-CRP score < 2.6 at month 6, with similar rates observed for all subgroups analysed according to prior therapy including those with prior JAK inhibitor exposure (range 57.4-71.0%), and in patients who received UPA monotherapy (71.6% [48/67]). Early (month 3) and sustained improvements up to 6 months were observed for all PROs. The safety profile was consistent with previous reports.
Conclusion: Real-world improvements in disease activity and PROs in response to UPA treatment were consistent with clinical trial data across a range of Canadian patients with prior therapy exposure and with UPA monotherapy, with an overall favourable benefit-risk profile.
Trial registration: NCT04574492.
Keywords: Clinical trial; Janus kinase inhibitor; Phase 4; Real-world effectiveness; Rheumatoid arthritis; Upadacitinib.
© 2024. The Author(s).
Conflict of interest statement
Louis Bessette reports consultancies, research grants, and speaker fees from BMS, Janssen, UCB, AbbVie, Pfizer, Sanofi, Lilly, and Novartis; research grants and speaker fees from Sandoz, Fresnius Kabi, Teva, Organon, and JAMP Pharma; and research grants from Gilead. Jonathan Chan reports consultancy and/or speaker fees and research grants from AbbVie, Pfizer, and UCB, and consultancy and/or speaker fees from Amgen, Celgene, Janssen, Novartis, Roche, Gilead, Eli Lilly, Merck, Fresenius Kabi, and Sandoz. Andrew Chow reports consultancies, research grants, and speaker fees from Abbvie, Amgen, AstraZeneca, BioJamp, Celltrion, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, UCB. Larissa Lisnevskaia has no conflicts to report. Nicolas Richard reports consultancies and/or speaker fees from Abbvie, Amgen, AstraZeneca, Eli Lilly, Janssen, Novartis, Pfizer and UCB. Pierre-Andre Fournier, Dalinda Liazoghli and Tanya Girard are employees of AbbVie Corporation. Derek Haaland reports honouraria from Abbvie, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi-Genzyme, Takeda, UCB; advisory board or speakers’ bureau membership for Abbvie, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda; research funding from Abbvie, Adiga Life Sciences, Amgen, AstraZeneca, Bristol-Myers Squibb, Can-Fite Biopharma, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, and UCB.
Figures
Similar articles
-
Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study.Arthritis Res Ther. 2025 Apr 10;27(1):84. doi: 10.1186/s13075-025-03528-5. Arthritis Res Ther. 2025. PMID: 40211417 Free PMC article.
-
Real-World Use and Effectiveness Outcomes in Patients with Rheumatoid Arthritis Treated with Upadacitinib: An Analysis from the CorEvitas Registry.Rheumatol Ther. 2024 Apr;11(2):363-380. doi: 10.1007/s40744-024-00639-4. Epub 2024 Feb 12. Rheumatol Ther. 2024. PMID: 38345715 Free PMC article.
-
Long-Term Efficacy and Safety of Upadacitinib in Patients with Rheumatoid Arthritis: Final Results from the BALANCE-EXTEND Open-Label Extension Study.Rheumatol Ther. 2023 Aug;10(4):901-915. doi: 10.1007/s40744-023-00557-x. Epub 2023 May 18. Rheumatol Ther. 2023. PMID: 37199884 Free PMC article.
-
Comparative efficacy of five approved Janus kinase inhibitors as monotherapy and combination therapy in patients with moderate-to-severe active rheumatoid arthritis: a systematic review and network meta-analysis of randomized controlled trials.Front Pharmacol. 2024 Apr 24;15:1387585. doi: 10.3389/fphar.2024.1387585. eCollection 2024. Front Pharmacol. 2024. PMID: 38725657 Free PMC article. Review.
-
Baricitinib as monotherapy for treatment of rheumatoid arthritis: analysis of real-world data.Curr Med Res Opin. 2024 Nov;40(11):1993-2002. doi: 10.1080/03007995.2024.2416979. Epub 2024 Oct 22. Curr Med Res Opin. 2024. PMID: 39436446 Review.
Cited by
-
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis.Rheumatol Ther. 2025 Feb;12(1):173-202. doi: 10.1007/s40744-024-00736-4. Epub 2025 Jan 6. Rheumatol Ther. 2025. PMID: 39757285 Free PMC article.
-
Real-World Experience With Janus Kinase Inhibitors in Immune-Mediated Diseases: Clinical Experience of a University Hospital.Cureus. 2024 Aug 25;16(8):e67729. doi: 10.7759/cureus.67729. eCollection 2024 Aug. Cureus. 2024. PMID: 39318929 Free PMC article.
-
Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study.Arthritis Res Ther. 2025 Apr 10;27(1):84. doi: 10.1186/s13075-025-03528-5. Arthritis Res Ther. 2025. PMID: 40211417 Free PMC article.
References
-
- Scott IC, Ibrahim F, Panayi G, et al. The frequency of remission and low disease activity in patients with rheumatoid arthritis, and their ability to identify people with low disability and normal quality of life. Semin Arthritis Rheum. 2019;49(1):20–26. doi: 10.1016/j.semarthrit.2018.12.006. - DOI - PubMed
-
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

