Impact of ITPA gene polymorphism for predicting anemia and treatment outcome in HCV infected patients taking Sofosbuvir Ribavirin therapy
- PMID: 38468199
- PMCID: PMC10926675
- DOI: 10.1186/s12879-024-09188-1
Impact of ITPA gene polymorphism for predicting anemia and treatment outcome in HCV infected patients taking Sofosbuvir Ribavirin therapy
Abstract
Background: Globally, 80 million people are suffering from chronic Hepatitis C virus (HCV) infection. Sofosbuvir ribavirin-based anti-HCV therapy is associated with anemia and other adverse effects. Polymorphisms of Inosine triphosphatase (ITPA) gene may cause functional impairment in the Inosine triphosphate pyrophosphatase enzyme, resulting in enhanced sustained viral response (SVR) and protection from ribavirin-associated anemia in patients on therapy. The study objective was to investigate the effect of Inosine triphosphatase gene polymorphism on SVR achievement, hemoglobin decline and ribavirin dose reduction in patients on therapy.
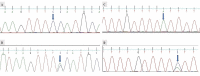
Methods: This prospective cohort study was of 170 hepatitis C infected patients received 6-month sofosbuvir ribavirin therapy. Patient viral load, reduction in ribavirin amount, liver function test, and complete blood count were noted monthly. Inosine triphosphatase variants rs1127354 and rs7270101 were assessed through the restriction fragment length polymorphism and confirmed using Sanger sequencing. The impact of polymorphism on cumulative reduction of ribavirin, and anti-HCV therapy outcome were studied.
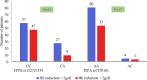
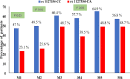
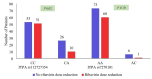
Results: A total of 74.3% of patients had ITPA rs1127354 CC genotype, 25.7% were CA and AA 0%. The frequency of ITPA genotype rs7270101-AA was 95%, AC 5%, and CC was 0%. ITPA rs1127354-CA had a notably positive impact on SVR achievement with a zero-relapse rate. ITPA rs1127354-CA genotype was significantly (P ˂0.05) protective against ≥ 2 g/dl Hb reduction from baseline to 1st, 2nd and 6th months of therapy. During treatment, Hb reduction ≥ 10 g/dl was frequently observed in rs1127354-CC genotype and rs7270101-AA genotype patients. Ribavirin dose reduction was significantly (P ˂0.05) high in rs1127354-CC genotype as compared to genotype CA whereas no significant difference was observed in ribavirin dose reduction in rs7270101 AA and non-AA genotype. Patient baseline characteristics such as age, body mass index, rs1127354-CC genotype, and baseline Hb were significantly associated with significant Hb reduction.
Conclusion: Pretreatment evaluation of ITPA polymorphism can be a diagnostic tool to find out patients at risk of anemia and improve treatment adherence. ITPA genotype rs1127354-CA contributes to improved compliance with ribavirin dose and protects against hemoglobin decline in HCV patients while taking ribavirin-based therapy. However, ITPA rs1127354, rs7270101 polymorphism have no significant impact on SVR achievement.
Keywords: Anemia; HCV; ITPA; Polymorphism; Ribavirin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Association of ITPA polymorphisms rs6051702/rs1127354 instead of rs7270101/rs1127354 as predictor of ribavirin-associated anemia in chronic hepatitis C treated patients.Antiviral Res. 2013 Oct;100(1):114-9. doi: 10.1016/j.antiviral.2013.07.021. Epub 2013 Aug 8. Antiviral Res. 2013. PMID: 23933495
-
Correlation between polymorphism in the inosine triphosphatase and the reductions in hemoglobin concentration and ribavirin dose during sofosbuvir and ribavirin therapy.J Gastroenterol Hepatol. 2017 Aug;32(8):1495-1502. doi: 10.1111/jgh.13743. J Gastroenterol Hepatol. 2017. PMID: 28109022
-
Relationship between ITPA polymorphisms and hemolytic anemia in HCV-infected patients after ribavirin-based therapy: a meta-analysis.J Transl Med. 2015 Oct 6;13:320. doi: 10.1186/s12967-015-0682-y. J Transl Med. 2015. PMID: 26438033 Free PMC article.
-
Association between inosine triphosphatase rs1127354 polymorphisms and ribavirin-induced anaemia and outcome in hepatitis C virus-infected patients: A meta-analysis.J Clin Pharm Ther. 2020 Dec;45(6):1218-1227. doi: 10.1111/jcpt.13232. Epub 2020 Jul 31. J Clin Pharm Ther. 2020. PMID: 32735044 Review.
-
Interleukin28B and inosine triphosphatase help to personalize hepatitis C treatment.Digestion. 2011;84 Suppl 1:50-5. doi: 10.1159/000333214. Epub 2011 Dec 2. Digestion. 2011. PMID: 22156486 Review.
References
-
- Jafar J, Junaid K, Saleem K, Amjed S, Arshad F, Amjed T, Raja N, Gillani A. Hypovitaminosis D in Hepatitis C patients and its relation with demographic and laboratory data. JPMA 2019. - PubMed
-
- Kozuka R, Hai H, Teranishi Y, Motoyama H, Kawamura E, Hagihara A, Uchida-Kobayashi S, Morikawa H, Enomoto M, Murakami Y. ITPA polymorphism correlates with the reductions in hemoglobin concentration and Ribavirin dose during sofosbuvir and Ribavirin therapy. J Gastroenterol Hepatol 2017. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

