Comparative effectiveness of implementation strategies for Accelerating Cervical Cancer Elimination through the integration of Screen-and-treat Services (ACCESS study): protocol for a cluster randomized hybrid type III trial in Nigeria
- PMID: 38468266
- PMCID: PMC10926605
- DOI: 10.1186/s13012-024-01349-9
Comparative effectiveness of implementation strategies for Accelerating Cervical Cancer Elimination through the integration of Screen-and-treat Services (ACCESS study): protocol for a cluster randomized hybrid type III trial in Nigeria
Abstract
Background: Despite the increased risk of cervical cancer (CC) among women living with HIV (WLHIV), CC screening and treatment (CCST) rates remain low in Africa. The integration of CCST services into established HIV programs in Africa can improve CC prevention and control. However, the paucity of evidence on effective implementation strategies (IS) has limited the success of integration in many countries. In this study, we seek to identify effective IS to enhance the integration of CCST services into existing HIV programs in Nigeria.
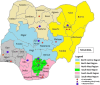
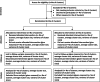
Methods: Our proposed study has formative and experimental activities across the four phases of the Exploration, Preparation, Implementation, and Sustainment (EPIS) framework. Through an implementation mapping conducted with stakeholders in the exploration phase, we identified a core package of IS (Core) and an enhanced package of IS (Core+) mostly selected from the Expert Recommendations for Implementing Change. In the preparation phase, we refined and tailored the Core and Core+ IS with the implementation resource teams for local appropriateness. In the implementation phase, we will conduct a cluster-randomized hybrid type III trial to assess the comparative effectiveness of Core versus Core+. HIV comprehensive treatment sites (k = 12) will be matched by region and randomized to Core or Core+ in the ratio of 1:1 stratified by region. In the sustainment phase, we will assess the sustainment of CCST at each site. The study outcomes will be assessed using RE-AIM: reach (screening rate), adoption (uptake of IS by study sites), IS fidelity (degree to which the IS occurred according to protocol), clinical intervention fidelity (delivery of CC screening, onsite treatment, and referral according to protocol), clinical effectiveness (posttreatment screen negative), and sustainment (continued integrated CCST service delivery). Additionally, we will descriptively explore potential mechanisms, including organizational readiness, implementation climate, CCST self-efficacy, and implementation intentions.
Discussion: The assessment of IS to increase CCST rates is consistent with the global plan of eliminating CC as a public health threat by 2030. Our study will identify a set of evidence-based IS for low-income settings to integrate evidence-based CCST interventions into routine HIV care in order to improve the health and life expectancy of WLHIV.
Trial registration: Prospectively registered on November 7, 2023, at ClinicalTrials.gov no. NCT06128304. https://classic.
Clinicaltrials: gov/ct2/show/study/NCT06128304.
Keywords: Africa; Cervical cancer; Implementation science; Nigeria; RE-AIM; Women living with HIV, EPIS framework, Exploration, Preparation, Implementation, and Sustainment framework.
© 2024. The Author(s).
Conflict of interest statement
GAA is a co-editor in chief at Implementation Science and is on the editorial board of Implementation Science Communications. All decisions on this paper were made by another editor. The other authors declare that they have no competing interests.
Figures
Similar articles
-
Rapid implementation mapping to identify implementation determinants and strategies for cervical cancer control in Nigeria.Front Public Health. 2023 Aug 17;11:1228434. doi: 10.3389/fpubh.2023.1228434. eCollection 2023. Front Public Health. 2023. PMID: 37663856 Free PMC article.
-
For girls and women (4GW) HPV RCT protocol: a crowdsourced, pragmatic stepped-wedge cluster randomized trial to improve uptake of HPV vaccination and screening among mother-daughter dyads in Nigeria.Implement Sci. 2025 May 1;20(1):18. doi: 10.1186/s13012-025-01428-5. Implement Sci. 2025. PMID: 40312377 Free PMC article.
-
Study protocol for a hybrid implementation-effectiveness trial of Game Changers for Cervical Cancer Prevention in Uganda.PLoS One. 2025 Jan 24;20(1):e0317491. doi: 10.1371/journal.pone.0317491. eCollection 2025. PLoS One. 2025. PMID: 39854403 Free PMC article.
-
Using the exploration, preparation, implementation, sustainment (EPIS) framework to assess the cooperative re-engagement controlled trial (CoRECT).Front Public Health. 2023 Dec 1;11:1223149. doi: 10.3389/fpubh.2023.1223149. eCollection 2023. Front Public Health. 2023. PMID: 38106893 Free PMC article. Review.
-
Leveraging the science of implementation: the case for specialized mental health community supervision.BMC Glob Public Health. 2025 Mar 31;3(1):27. doi: 10.1186/s44263-025-00147-9. BMC Glob Public Health. 2025. PMID: 40159465 Free PMC article. Review.
Cited by
-
Evaluating the tailored implementation of a multisite care navigation service for mental health in rural and remote Australia (The Bridging Study): protocol for a community-engaged hybrid effectiveness-implementation study.Implement Sci. 2024 Sep 4;19(1):62. doi: 10.1186/s13012-024-01391-7. Implement Sci. 2024. PMID: 39232820 Free PMC article.
References
-
- World Health Organization . Africa: Global Cancer Observatory. 2021.
-
- International Agency for Research on Cancer . Cancer Tomorrow. 2023.