Salvianolic acid C attenuates cerebral ischemic injury through inhibiting neuroinflammation via the TLR4-TREM1-NF-κB pathway
- PMID: 38468280
- PMCID: PMC10929175
- DOI: 10.1186/s13020-024-00914-0
Salvianolic acid C attenuates cerebral ischemic injury through inhibiting neuroinflammation via the TLR4-TREM1-NF-κB pathway
Abstract
Background: Stroke is a leading cause of mortality and disability with ischemic stroke being the most common type of stroke. Salvianolic acid C (SalC), a polyphenolic compound found in Salviae Miltiorrhizae Radix et Rhizoma, has demonstrated therapeutic potential in the recovery phase of ischemic stroke. However, its pharmacological effects and underlying mechanisms during the early stages of ischemic stroke remain unclear. This study aimed to examine the potential mechanism of action of SalC during the early phase of ischemic stroke using network pharmacology strategies and RNA sequencing analysis.
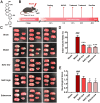
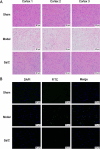
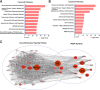
Methods: SalC effects on infarct volume, neurological deficits, and histopathological changes were assessed in a mouse model of transient middle cerebral artery occlusion (tMCAO). By integrating RNA sequencing data with a cerebral vascular disease (CVD)-related gene database, a cerebral ischemic disease (CID) network containing dysregulated genes from the tMCAO model was constructed. Network analysis algorithms were applied to evaluate the key nodes within the CID network. In vivo and in vitro validation of crucial targets within the identified pathways was conducted.
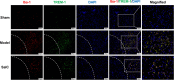
Results: SalC treatment significantly reduced infarct volume, improved neurological deficits, and reversed pathological changes in the tMCAO mouse model. The integration of RNA sequencing data revealed an 80% gene reversion rate induced by SalC within the CID network. Among the reverted genes, 53.1% exhibited reversion rates exceeding 50%, emphasizing the comprehensive rebalancing effect of SalC within the CID network. Neuroinflammatory-related pathways regulated by SalC, including the toll-like-receptor 4 (TLR4)- triggering receptor expressed on myeloid cells 1 (TREM1)-nuclear factor kappa B (NF-κB) pathway, were identified. Further in vivo and in vitro experiments confirmed that TLR4-TREM1-NF-κB pathway was down-regulated by SalC in microglia, which was essential for its anti-inflammatory effect on ischemic stroke.
Conclusions: SalC attenuated cerebral ischemic injury by inhibiting neuroinflammation mediated by microglia, primarily through the TLR4-TREM1-NF-κB pathway. These findings provide valuable insights into the potential therapeutic benefits of SalC in ischemic stroke.
Keywords: Cerebral ischemic stroke; Disease network; Neuroinflammation; RNA transcriptome sequencing; Salvianolic acid C; TREM1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
-
- Hlavica M, Diepers M, Garcia-Esperon C, Ineichen BV, Nedeltchev K, Kahles T, Remonda L. Pharmacological recanalization therapy in acute ischemic stroke—evolution, current state and perspectives of intravenous and intra-arterial thrombolysis. J Neuroradiol. 2015;42(1):30–46. doi: 10.1016/j.neurad.2014.11.004. - DOI - PubMed
Grants and funding
- 2024C03106/the "Pioneer" and "Leading Goose" R&D Program of Zhejiang Province
- ZYYCXTD-D-202002/Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine
- 226-2023-00114/Fundamental Research Funds for Central Universities of the Central South University
LinkOut - more resources
Full Text Sources

