Differentiation is accompanied by a progressive loss in transcriptional memory
- PMID: 38468285
- PMCID: PMC10929117
- DOI: 10.1186/s12915-024-01846-9
Differentiation is accompanied by a progressive loss in transcriptional memory
Abstract
Background: Cell differentiation requires the integration of two opposite processes, a stabilizing cellular memory, especially at the transcriptional scale, and a burst of gene expression variability which follows the differentiation induction. Therefore, the actual capacity of a cell to undergo phenotypic change during a differentiation process relies upon a modification in this balance which favors change-inducing gene expression variability. However, there are no experimental data providing insight on how fast the transcriptomes of identical cells would diverge on the scale of the very first two cell divisions during the differentiation process.
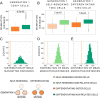
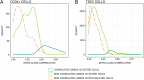
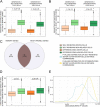
Results: In order to quantitatively address this question, we developed different experimental methods to recover the transcriptomes of related cells, after one and two divisions, while preserving the information about their lineage at the scale of a single cell division. We analyzed the transcriptomes of related cells from two differentiation biological systems (human CD34+ cells and T2EC chicken primary erythrocytic progenitors) using two different single-cell transcriptomics technologies (scRT-qPCR and scRNA-seq).
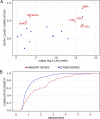
Conclusions: We identified that the gene transcription profiles of differentiating sister cells are more similar to each other than to those of non-related cells of the same type, sharing the same environment and undergoing similar biological processes. More importantly, we observed greater discrepancies between differentiating sister cells than between self-renewing sister cells. Furthermore, a progressive increase in this divergence from first generation to second generation was observed when comparing differentiating cousin cells to self renewing cousin cells. Our results are in favor of a gradual erasure of transcriptional memory during the differentiation process.
Keywords: Cell differentiation; Gene expression variability; Sister cells; Transcriptional memory; Transcriptome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

