APOE4 genotype and aging impair injury-induced microglial behavior in brain slices, including toward Aβ, through P2RY12
- PMID: 38468308
- PMCID: PMC10929239
- DOI: 10.1186/s13024-024-00714-y
APOE4 genotype and aging impair injury-induced microglial behavior in brain slices, including toward Aβ, through P2RY12
Abstract
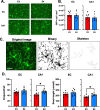
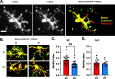
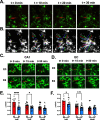
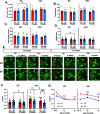
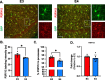
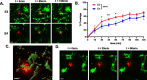
Microglia are highly dynamic cells that play a critical role in tissue homeostasis through the surveillance of brain parenchyma and response to cues associated with damage. Aging and APOE4 genotype are the strongest risk factors for Alzheimer's disease (AD), but how they affect microglial dynamics remains unclear. Using ex vivo confocal microscopy, we analyzed microglial dynamic behaviors in the entorhinal cortex (EC) and hippocampus CA1 of 6-, 12-, and 21-month-old mice APOE3 or APOE4 knock-in mice expressing GFP under the CX3CR1 promoter. To study microglia surveillance, we imaged microglia baseline motility for 20 min and measured the extension and retraction of processes. We found that APOE4 microglia exhibited significantly less brain surveillance (27%) compared to APOE3 microglia in 6-month-old mice; aging exacerbated this deficit. To measure microglia response to damage, we imaged process motility in response to ATP, an injury-associated signal, for 30 min. We found APOE4 microglia extended their processes significantly slower (0.9 µm/min, p < 0.005) than APOE3 microglia (1.1 μm/min) in 6-month-old animals. APOE-associated alterations in microglia motility were observed in 12- and 21-month-old animals, and this effect was exacerbated with aging in APOE4 microglia. We measured protein and mRNA levels of P2RY12, a core microglial receptor required for process movement in response to damage. We found that APOE4 microglia express significantly less P2RY12 receptors compared to APOE3 microglia despite no changes in P2RY12 transcripts. To examine if the effect of APOE4 on the microglial response to ATP also applied to amyloid β (Aβ), we infused locally Hi-Lyte Fluor 555-labeled Aβ in acute brain slices of 6-month-old mice and imaged microglia movement for 2 h. APOE4 microglia showed a significantly slower (p < 0.0001) process movement toward the Aβ, and less Aβ coverage at early time points after Aβ injection. To test whether P2RY12 is involved in process movement in response to Aβ, we treated acute brain slices with a P2RY12 antagonist before Aβ injection; microglial processes no longer migrated towards Aβ. These results provide mechanistic insights into the impact of APOE4 genotype and aging in dynamic microglial behaviors prior to gross Aβ pathology and could help explain how APOE4 brains are more susceptible to AD pathogenesis.
Keywords: Ex-vivo imaging; APOE4; Aging; Alzheimer’s disease; Microglia; P2RY12.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing interests.
Figures
References
-
- 2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4), 700–789. 10.1002/alz.12638. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

