In-depth proteome characterization of endometrium and extraembryonic membranes during implantation in pig
- PMID: 38468318
- PMCID: PMC10929143
- DOI: 10.1186/s40104-024-01002-x
In-depth proteome characterization of endometrium and extraembryonic membranes during implantation in pig
Abstract
Background: Proteome characterization of the porcine endometrium and extraembryonic membranes is important to understand mother-embryo cross-communication. In this study, the proteome of the endometrium and chorioallantoic membrane was characterized in pregnant sows (PS) during early gestation (d 18 and 24 of gestation) and in the endometrium of non-pregnant sows (NPS) during the same days using LC-MS/MS analysis. The UniProtKB database and ClueGO were used to obtain functional Gene Ontology annotations and biological and functional networks, respectively.
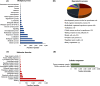
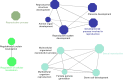
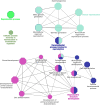
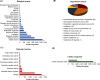
Results: Our analysis yielded 3,254 and 3,457 proteins identified in the endometrium of PS and NPS, respectively; of these, 1,753 being common while 1,501 and 1,704 were exclusive to PS and NPS, respectively. In addition, we identified 3,968 proteins in the extraembryonic membranes of PS. Further analyses of function revealed some proteins had relevance for the immune system process and biological adhesion in endometrium while the embryonic chorion displayed abundance of proteins related to cell adhesion and cytoskeletal organization, suggesting they dominated the moment of endometrial remodeling, implantation and adhesion of the lining epithelia. Data are available via ProteomeXchange with identifier PXD042565.
Conclusion: This is the first in-depth proteomic characterization of the endometrium and extraembryonic membranes during weeks 3 to 4 of gestation; data that contribute to the molecular understanding of the dynamic environment during this critical period, associated with the majority of pregnancy losses.
Keywords: Endometrium; Extraembryonic membranes; Implantation; Pig; Proteome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Pope WF, First NL. Factors affecting the survival of pig embryos. Theriogenology. 1985;23(1):91–105. doi: 10.1016/0093-691X(85)90075-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

