Microbiome changes through the ontogeny of the marine sponge Crambe crambe
- PMID: 38468324
- PMCID: PMC10929144
- DOI: 10.1186/s40793-024-00556-7
Microbiome changes through the ontogeny of the marine sponge Crambe crambe
Abstract
Background: Poriferans (sponges) are highly adaptable organisms that can thrive in diverse marine and freshwater environments due, in part, to their close associations with internal microbial communities. This sponge microbiome can be acquired from the surrounding environment (horizontal acquisition) or obtained from the parents during the reproductive process through a variety of mechanisms (vertical transfer), typically resulting in the presence of symbiotic microbes throughout all stages of sponge development. How and to what extent the different components of the microbiome are transferred to the developmental stages remain poorly understood. Here, we investigated the microbiome composition of a common, low-microbial-abundance, Atlantic-Mediterranean sponge, Crambe crambe, throughout its ontogeny, including adult individuals, brooded larvae, lecithotrophic free-swimming larvae, newly settled juveniles still lacking osculum, and juveniles with a functional osculum for filter feeding.
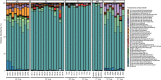
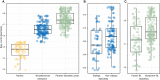
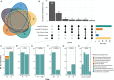
Results: Using 16S rRNA gene analysis, we detected distinct microbiome compositions in each ontogenetic stage, with variations in composition, relative abundance, and diversity of microbial species. However, a particular dominant symbiont, Candidatus Beroebacter blanensis, previously described as the main symbiont of C. crambe, consistently occurred throughout all stages, an omnipresence that suggests vertical transmission from parents to offspring. This symbiont fluctuated in relative abundance across developmental stages, with pronounced prevalence in lecithotrophic stages. A major shift in microbial composition occurred as new settlers completed osculum formation and acquired filter-feeding capacity. Candidatus Beroebacter blanensis decreased significatively at this point. Microbial diversity peaked in filter-feeding stages, contrasting with the lower diversity of lecithotrophic stages. Furthermore, individual specific transmission patterns were detected, with greater microbial similarity between larvae and their respective parents compared to non-parental conspecifics.
Conclusions: These findings suggest a putative vertical transmission of the dominant symbiont, which could provide some metabolic advantage to non-filtering developmental stages of C. crambe. The increase in microbiome diversity with the onset of filter-feeding stages likely reflects enhanced interaction with environmental microbes, facilitating horizontal transmission. Conversely, lower microbiome diversity in lecithotrophic stages, prior to filter feeding, suggests incomplete symbiont transfer or potential symbiont digestion. This research provides novel information on the dynamics of the microbiome through sponge ontogeny, on the strategies for symbiont acquisition at each ontogenetic stage, and on the potential importance of symbionts during larval development.
Keywords: 16S rRNA gene; Demospongiae; Life cycle; Porifera; Symbionts; Vertical transmission.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
The chromosomal genome sequence of the sponge Crambe crambe (Schmidt, 1862) and its associated microbial metagenome sequences.Wellcome Open Res. 2025 May 23;10:275. doi: 10.12688/wellcomeopenres.24154.1. eCollection 2025. Wellcome Open Res. 2025. PMID: 40520149 Free PMC article.
-
Stable microbial communities in the sponge Crambe crambe from inside and outside a polluted Mediterranean harbor.FEMS Microbiol Lett. 2017 Jun 15;364(11). doi: 10.1093/femsle/fnx105. FEMS Microbiol Lett. 2017. PMID: 28520957
-
High microbiome and metabolome diversification in coexisting sponges with different bio-ecological traits.Commun Biol. 2024 Apr 8;7(1):422. doi: 10.1038/s42003-024-06109-5. Commun Biol. 2024. PMID: 38589605 Free PMC article.
-
Marine sponge microbial association: Towards disclosing unique symbiotic interactions.Mar Environ Res. 2018 Sep;140:169-179. doi: 10.1016/j.marenvres.2018.04.017. Epub 2018 Apr 27. Mar Environ Res. 2018. PMID: 29935729 Review.
-
Symbiont transmission in marine sponges: reproduction, development, and metamorphosis.BMC Biol. 2022 May 6;20(1):100. doi: 10.1186/s12915-022-01291-6. BMC Biol. 2022. PMID: 35524305 Free PMC article. Review.
Cited by
-
Epibiotic bacterial community composition varies during different developmental stages of Octopus mimus: Study of cultivable representatives and their secondary metabolite production.PLoS One. 2024 Dec 30;19(12):e0312991. doi: 10.1371/journal.pone.0312991. eCollection 2024. PLoS One. 2024. PMID: 39775278 Free PMC article.
-
The chromosomal genome sequence of the sponge Crambe crambe (Schmidt, 1862) and its associated microbial metagenome sequences.Wellcome Open Res. 2025 May 23;10:275. doi: 10.12688/wellcomeopenres.24154.1. eCollection 2025. Wellcome Open Res. 2025. PMID: 40520149 Free PMC article.
-
Marine Mycobiomes Colonize Mediterranean Sponge Hosts in a Random Fashion.Microb Ecol. 2025 Apr 10;88(1):25. doi: 10.1007/s00248-025-02523-2. Microb Ecol. 2025. PMID: 40208324 Free PMC article.
-
Dynamics, diversity, and roles of bacterial transmission modes during the first asexual life stages of the freshwater sponge Spongilla lacustris.Environ Microbiome. 2024 Jun 8;19(1):37. doi: 10.1186/s40793-024-00580-7. Environ Microbiome. 2024. PMID: 38851755 Free PMC article.
-
Connectivity and Adaptation Patterns of the Deep-Sea Ground-Forming Sponge Geodia hentscheli Across Its Entire Distribution.Mol Biol Evol. 2025 Jul 1;42(7):msaf145. doi: 10.1093/molbev/msaf145. Mol Biol Evol. 2025. PMID: 40476758 Free PMC article.
References
-
- Wulff J. Ecological interactions and the distribution, abundance, and diversity of sponges. In: Advances in marine biology. Elsevier; 2012. p. 273–344. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
