Dual Functional Materials: At the Interface of Catalysis and Separations
- PMID: 38468456
- PMCID: PMC11100017
- DOI: 10.1021/acs.langmuir.3c03888
Dual Functional Materials: At the Interface of Catalysis and Separations
Abstract
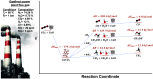
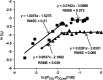
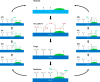
Dual functional materials (DFMs) are a promising approach to increase the energy efficiency of carbon capture and utilization by combining both steps into a single unit operation. In this Perspective, we analyze the challenges and opportunities of integrated carbon capture and utilization (ICCU) via a thermally driven process. We identify three key areas that will facilitate research progress toward industrially viable solutions: (1) selecting appropriate DFM operating conditions; (2) designing and characterizing interfacial site cooperativity for CO2 adsorption and hydrogenation; and (3) establishing standards for rigorous and comprehensive data reporting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Integrated CO2 capture and reverse water-gas shift reaction over CeO2-CaO dual functional materials.R Soc Open Sci. 2023 Apr 5;10(4):230067. doi: 10.1098/rsos.230067. eCollection 2023 Apr. R Soc Open Sci. 2023. PMID: 37035291 Free PMC article.
-
Enhancement of the CO2 adsorption and hydrogenation to CH4 capacity of Ru-Na-Ca/γ-Al2O3 dual function material by controlling the Ru calcination atmosphere.J Environ Sci (China). 2024 Jun;140:292-305. doi: 10.1016/j.jes.2023.08.041. Epub 2023 Sep 14. J Environ Sci (China). 2024. PMID: 38331509
-
The Effect of Alkali Metals (Li, Na, and K) on Ni/CaO Dual-Functional Materials for Integrated CO2 Capture and Hydrogenation.Materials (Basel). 2023 Aug 2;16(15):5430. doi: 10.3390/ma16155430. Materials (Basel). 2023. PMID: 37570134 Free PMC article.
-
Current and future perspectives on catalytic-based integrated carbon capture and utilization.Sci Total Environ. 2021 Oct 10;790:148081. doi: 10.1016/j.scitotenv.2021.148081. Epub 2021 May 27. Sci Total Environ. 2021. PMID: 34091328 Review.
-
Recent Progress in the Integration of CO2 Capture and Utilization.Molecules. 2023 Jun 1;28(11):4500. doi: 10.3390/molecules28114500. Molecules. 2023. PMID: 37298975 Free PMC article. Review.
Cited by
-
A review of large language models and autonomous agents in chemistry.Chem Sci. 2024 Dec 9;16(6):2514-2572. doi: 10.1039/d4sc03921a. eCollection 2025 Feb 5. Chem Sci. 2024. PMID: 39829984 Free PMC article. Review.
References
-
- Sun S.; Sun H.; Williams P. T.; Wu C. Recent advances in integrated CO2 capture and utilization: a review. Sustainable Energy & Fuels 2021, 5 (18), 4546–4559. 10.1039/D1SE00797A. - DOI
-
- Shao B.; Hu G.; Alkebsi K. A. M.; Ye G.; Lin X.; Du W.; Hu J.; Wang M.; Liu H.; Qian F. Heterojunction-redox catalysts of FexCoyMg10CaO for high-temperature CO2 capture and in situ conversion in the context of green manufacturing. Energy Environ. Sci. 2021, 14 (4), 2291–2301. 10.1039/D0EE03320K. - DOI
-
- Schmitt T.; Leptinsky S.; Turner M.; Zoelle A.; White C. W.; Hughes S.; Homsy S.; Woods M.; Hoffman H.; Shultz T.; James Iii R. E. Cost and Performance Baseline for Fossil Energy Plants Volume 1: Bituminous Coal and Natural Gas to Electricity. U.S. Department of Energy, Office of Scientific and Technical Information 2022, 10.2172/1893822. - DOI
-
- U.S. Environmental Protection Agency . AP-42, Fifth Edition Compilation of Air Pollutant Emissions Factors, Volume 1: Stationary Point and Area Sources.http://www.epa.gov/air-emissions-factors-and-quantification/ap-42-compil....
Publication types
LinkOut - more resources
Full Text Sources

