CD4+ CD11b+ T cells infiltrate and aggravate the traumatic brain injury depending on brain-to-cervical lymph node signaling
- PMID: 38468459
- PMCID: PMC10928342
- DOI: 10.1111/cns.14673
CD4+ CD11b+ T cells infiltrate and aggravate the traumatic brain injury depending on brain-to-cervical lymph node signaling
Abstract
Aim: We aim to identify the specific CD4+ T-cell subtype influenced by brain-to-CLN signaling and explore their role during the acute phase of traumatic brain injury (TBI).
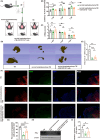
Method: Cervical lymphadenectomy or cervical afferent lymphatic ligation was performed before TBI. Cytokine array and western blot were used to detect cytokines, while the motor function was assessed using mNss and rotarod test. CD4+ T-cell subtypes in blood, brain, and CLNs were analyzed with Cytometry by time-of-flight analysis (CyTOF) or fluorescence-activated cell sorting (FACS). Brain edema and volume changes were measured by 9.4T MRI. Neuronal apoptosis was evaluated by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining.
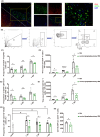
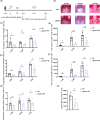
Results: Cervical lymphadenectomy and ligation of cervical lymphatic vessels resulted in a decreased infiltration of CD4+ T cells, specifically CD11b-positive CD4+ T cells, within the affected region. The population of CD4+ CD11b+ T cells increased in ligated CLNs, accompanied by a decrease in the average fluorescence intensity of sphingosine-1-phosphate receptor-1 (S1PR1) on these cells. Administration of CD4+ CD11b+ T cells sorted from CLNs into the lateral ventricle reversed the attenuated neurologic deficits, brain edema, and lesion volume following cervical lymphadenectomy.
Conclusion: The infiltration of CD4+ CD11b+ T cells exacerbates secondary brain damage in TBI, and this process is modulated by brain-to-CLN signaling.
Keywords: CD4+ T cell; brain-to-cervical lymph node signaling; immunoregulation; traumatic brain injury.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury.J Neuroinflammation. 2017 Aug 23;14(1):167. doi: 10.1186/s12974-017-0934-2. J Neuroinflammation. 2017. PMID: 28835272 Free PMC article.
-
Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway.Drug Des Devel Ther. 2018 Aug 10;12:2497-2508. doi: 10.2147/DDDT.S163951. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30127597 Free PMC article.
-
FTY720 Reduces Endothelial Cell Apoptosis and Remodels Neurovascular Unit after Experimental Traumatic Brain Injury.Int J Med Sci. 2021 Jan 1;18(2):304-313. doi: 10.7150/ijms.49066. eCollection 2021. Int J Med Sci. 2021. PMID: 33390799 Free PMC article.
-
Follicular Dendritic Cells of Lymph Nodes as Human Immunodeficiency Virus/Simian Immunodeficiency Virus Reservoirs and Insights on Cervical Lymph Node.Front Immunol. 2018 Apr 19;9:805. doi: 10.3389/fimmu.2018.00805. eCollection 2018. Front Immunol. 2018. PMID: 29725333 Free PMC article. Review.
-
B-Cell Lymphoma 2 (Bcl-2) and Regulation of Apoptosis after Traumatic Brain Injury: A Clinical Perspective.Medicina (Kaunas). 2020 Jun 18;56(6):300. doi: 10.3390/medicina56060300. Medicina (Kaunas). 2020. PMID: 32570722 Free PMC article.
Cited by
-
Editorial: Neurodegeneration and cognitive impairment after traumatic brain injury.Front Neurosci. 2025 Jan 29;19:1552527. doi: 10.3389/fnins.2025.1552527. eCollection 2025. Front Neurosci. 2025. PMID: 39944890 Free PMC article. No abstract available.
-
Enhancing daily living and cognitive functions in traumatic brain injury patients through Orem's self-care theory.Front Neurol. 2024 Aug 20;15:1449417. doi: 10.3389/fneur.2024.1449417. eCollection 2024. Front Neurol. 2024. PMID: 39228512 Free PMC article.
-
Neuroinflammation and acute ischemic stroke: impact on translational research and clinical care.Front Surg. 2025 Apr 28;12:1501359. doi: 10.3389/fsurg.2025.1501359. eCollection 2025. Front Surg. 2025. PMID: 40356948 Free PMC article. Review.
-
CSF transport at the brain-meningeal border: effects on neurological health and disease.Lancet Neurol. 2025 Jun;24(6):535-547. doi: 10.1016/S1474-4422(25)00115-2. Lancet Neurol. 2025. PMID: 40409317 Review.
-
The Cerebral Lymphatic System: Function, Controversies, and Therapeutic Approaches for Central Nervous System Diseases.Cell Mol Neurobiol. 2025 Aug 19;45(1):80. doi: 10.1007/s10571-025-01598-2. Cell Mol Neurobiol. 2025. PMID: 40828342 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

