Safety assessment of equine allogeneic tenogenic primed mesenchymal stem cells in horses with naturally occurring tendon and ligament injuries
- PMID: 38468694
- PMCID: PMC10925754
- DOI: 10.3389/fvets.2024.1282697
Safety assessment of equine allogeneic tenogenic primed mesenchymal stem cells in horses with naturally occurring tendon and ligament injuries
Abstract
Background: Mesenchymal stem cells provide a valuable treatment option in orthopedic injuries in horses.
Objectives: The aim of this study was to evaluate the hematological, biochemical, immunological and immunomodulatory parameters following intralesional treatment with tenogenic primed equine allogeneic peripheral blood-derived mesenchymal stem cells (tpMSCs) in client-owned horses with naturally occurring superficial digital flexor tendon (SDFT) and suspensory ligament (SL) injuries.
Methods: The immunogenicity and immunomodulatory capacities of tpMSCs were assessed in a modified mixed lymphocyte reaction, including peripheral blood mononuclear cells (PBMCs) of 14 horses with SDFT and SL injuries after treatment with tpMSCs. In a second study, 18 horses with SDFT and SL injuries received either an intralesional injection with tpMSCs (n = 9) or no treatment (n = 9).
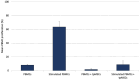
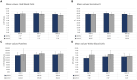
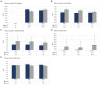
Results: The tpMSCs did not provoke a cellular immune response (p < 0.001) and were able to immunomodulate stimulated T lymphocytes (p < 0.001) in vitro. Therapeutic use of tpMSCs did not result in relevant hematologic or biochemical abnormalities.
Main limitations: Both studies had a small sample size. No statistical analyses were performed in the second study. Fibrinogen was only analyzed in a single horse prior to treatment.
Conclusion: Co-incubation of tpMSCs and PBMCs of horses that have been previously exposed to tpMSCs did not elicit a cellular immune response and tpMSCs were able to immunomodulate stimulated T lymphocytes. Intralesional treatment with tpMSCs did not provoke abnormal changes in hematological and biochemical parameters.
Keywords: desmitis; equine; mesenchymal stem cell; primed MSCs; tendonitis.
Copyright © 2024 Carlier, Depuydt, Van Hecke, Martens, Saunders and Spaas.
Conflict of interest statement
SC, ED, LV, and JHS were employed by Boehringer-Ingelheim or an affiliated company at the time of the study. The content of this manuscript contains a commercially available stem cell product (RenuTend©) owned and patented by Boehringer-Ingelheim. The funder had the following involvement in the study: the studies were performed to collect additional data for Marketing Authorization. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. One of the authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Geburek F, Roggel F, Van Schie HTM, Beineke A, Estrada R, Weber K, et al. . Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. (2017) 8:129. doi: 10.1186/s13287-017-0564-8, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

