Characterizing oogenesis and programmed cell death in the eastern tree hole mosquito Aedes (Protomacleaya) triseriatus
- PMID: 38468807
- PMCID: PMC10926484
- DOI: 10.3389/finsc.2022.1073308
Characterizing oogenesis and programmed cell death in the eastern tree hole mosquito Aedes (Protomacleaya) triseriatus
Abstract
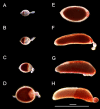
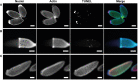
Oogenesis in flies manifests as a carefully orchestrated cascade of developmental gates and growth events, punctuated by programmed cell death (PCD) and follicular resorption events. In anautogenous mosquitoes, a blood meal stimulates growth of primary follicles, but the timing of developmental stages is species-specific, and few species have been characterized. Here, we characterize the first gonotrophic cycle of oogenesis in Aedes triseriatus (Diptera: Culicidae), the principal vector of La Crosse Virus (LACV), a major cause of pediatric encephalitis in North America. We note significant differences in the timing and appearance of developmental stages from previous studies of other mosquito species, particularly Aedes aegypti. We also describe the appearance and timing of PCD events including atresia, nurse cell death, and follicular epithelium death and show that the majority of follicular epithelium cells do not undergo apoptosis during oogenesis but persist in the ovariole at least until the second gonotrophic cycle. This thorough characterization of oogenesis and PCD in Ae. triseriatus, through which LACV must persist in order to achieve filial infection, also serves as a baseline to study host-pathogen interactions during transovarial transmission.
Keywords: apoptosis; atresia; autophagy; nurse cell death; ovarian development.
Copyright © 2023 Airs, Nazarchyk, Tucker and Bartholomay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Detection of La Crosse Virus In Situ and in Individual Progeny to Assess the Vertical Transmission Potential in Aedes albopictus and Aedes aegypti.Insects. 2023 Jul 3;14(7):601. doi: 10.3390/insects14070601. Insects. 2023. PMID: 37504607 Free PMC article.
-
Comparative Susceptibility of Ochlerotatus japonicus, Ochlerotatus triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to La Crosse Virus.J Med Entomol. 2016 Nov;53(6):1415-1421. doi: 10.1093/jme/tjw097. Epub 2016 Sep 7. J Med Entomol. 2016. PMID: 27605372
-
Midgut and salivary gland barriers to La Crosse virus dissemination in mosquitoes of the Aedes triseriatus group.Med Vet Entomol. 1989 Apr;3(2):113-23. doi: 10.1111/j.1365-2915.1989.tb00485.x. Med Vet Entomol. 1989. PMID: 2519653
-
Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence.Ecohealth. 2012 Jun;9(2):217-28. doi: 10.1007/s10393-012-0773-7. Epub 2012 Jun 13. Ecohealth. 2012. PMID: 22692799 Free PMC article. Review.
-
[Morphofunctional changes of the ovarioles of blood-sucking mosquitoes (Diptera, Culicidae) during oogenesis. 2. Abortive oogenesis].Med Parazitol (Mosk). 1989 May-Jun;(3):55-60. doi: 10.21236/ada227408. Med Parazitol (Mosk). 1989. PMID: 2571065 Review. Russian.
References
-
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of north America, north of Mexico. Gainesville, Florida, USA: University Press of Florida; (2005).
LinkOut - more resources
Full Text Sources

