JAG1 Variants Confer Genetic Susceptibility to Thyroid Dysgenesis and Thyroid Dyshormonogenesis in 813 Congenital Hypothyroidism in China
- PMID: 38468821
- PMCID: PMC10926855
- DOI: 10.2147/IJGM.S445557
JAG1 Variants Confer Genetic Susceptibility to Thyroid Dysgenesis and Thyroid Dyshormonogenesis in 813 Congenital Hypothyroidism in China
Abstract
Background and objective: Congenital hypothyroidism (CH) is indeed a prevalent neonatal endocrine disorder, affecting approximately 1 in 2000-3000 newborns worldwide, and 1 in 2400 newborns in China. Despite its high incidence, the genetic causes of CH, particularly those related to thyroid dysgenesis (TD), are still not well understood. However, previous studies have suggested that JAG1 may be a potential susceptibility gene for congenital thyroid defects. To explore the association between JAG1 and CH, we screened JAG1 variants in a large cohort of 813 CH patients.
Methods: We performed genetic analysis of JAG1 using next-generation sequencing in 813 CH cases. The pathogenicity of the variants was assessed by bioinformatics softwares, protein sequence conservation analysis, and hydrophobic analysis. Further genetic analysis was conducted targeting 20 CH-related genes in these 25 JAG1 variant carriers.
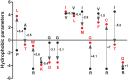
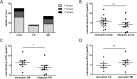
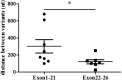
Results: We identified 10 pathogenic missense mutations (p.V45L, p.V272I, p.P552L, p.G610E, p.G852D, p.A891T, p.E1030K, p.R1060W, p.A1131T, p.P1174L) carried by 25 patients, the mutation rate of JAG1 in CH was 3.08%. Among these 25 patients, 16 with 1 variant, 6 with 2 variants, and the other 3 with 3 variants. Our findings indicated that JAG1 variants confer genetic susceptibility to both TD and DH, but with different inheritance models. JAG1 variants lead to TD mainly through monogenic model, while for DH cases, both monogenic mechanisms and oligogenic mechanisms play a pivotal role. Oligogenicity may contribute to the disease severity of DH.
Conclusion: JAG1 is a shared genetic factor in TD and DH, with a detection rate of 3.08% in Chinese individuals with CH. A comparison between the oligogenic and monogenic groups suggests a gene dosage effect in CH. Patients with the same JAG1 mutation exhibit diverse clinical phenotypes, indicating complex mechanisms underlying phenotypic heterogeneity.
Keywords: JAG1; congenital hypothyroidism; inheritance model; pathogenic variant; phenotypic heterogeneity.
© 2024 Li et al.
Conflict of interest statement
The authors declare no potential conflicts of interest in this work.
Figures

Similar articles
-
The mutation screening in candidate genes related to thyroid dysgenesis by targeted next-generation sequencing panel in the Chinese congenital hypothyroidism.Clin Endocrinol (Oxf). 2022 Apr;96(4):617-626. doi: 10.1111/cen.14577. Epub 2021 Aug 9. Clin Endocrinol (Oxf). 2022. PMID: 34374102
-
Mutation screening of eight genes and comparison of the clinical data in a Chinese cohort with congenital hypothyroidism.Endocrine. 2023 Jan;79(1):125-134. doi: 10.1007/s12020-022-03188-4. Epub 2022 Sep 20. Endocrine. 2023. PMID: 36125728
-
Targeted Next-Generation Sequencing for Congenital Hypothyroidism With Positive Neonatal TSH Screening.J Clin Endocrinol Metab. 2020 Aug 1;105(8):dgaa308. doi: 10.1210/clinem/dgaa308. J Clin Endocrinol Metab. 2020. PMID: 32459320
-
New genetics in congenital hypothyroidism.Endocrine. 2021 Mar;71(3):696-705. doi: 10.1007/s12020-021-02646-9. Epub 2021 Mar 1. Endocrine. 2021. PMID: 33650047 Review.
-
Genetics of congenital hypothyroidism: Modern concepts.Pediatr Investig. 2022 May 14;6(2):123-134. doi: 10.1002/ped4.12324. eCollection 2022 Jun. Pediatr Investig. 2022. PMID: 35774517 Free PMC article. Review.
Cited by
-
Personalized Management of Malignant and Non-Malignant Ectopic Mediastinal Thyroid: A Proposed 10-Item Algorithm Approach.Cancers (Basel). 2024 May 14;16(10):1868. doi: 10.3390/cancers16101868. Cancers (Basel). 2024. PMID: 38791947 Free PMC article. Review.
References
-
- van Trotsenburg P, Stoupa A, Leger J, et al. Congenital Hypothyroidism: a 2020-2021 Consensus Guidelines Update-An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021;31(3):387–419. doi:10.1089/thy.2020.0333 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

