A comparison of the time course of action and laryngeal mask airway insertion conditions with different doses of mivacurium for day-case urologic surgery in children: a prospective cohort study
- PMID: 38468874
- PMCID: PMC10925757
- DOI: 10.3389/fped.2024.1330737
A comparison of the time course of action and laryngeal mask airway insertion conditions with different doses of mivacurium for day-case urologic surgery in children: a prospective cohort study
Abstract
Objective: To investigate the time course of action of different doses of mivacurium and determine the appropriate dose for laryngeal mask airway (LMA) insertion for day-case urologic surgery in children.
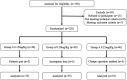
Methods: A total of 105 patients who enrolled in this study between March 2021 and December 2021 were randomised into 3 groups: Group A (mivacurium 0.15 mg/kg, n = 35), Group B (mivacurium 0.20 mg/kg, n = 35) and Group C (mivacurium 0.25 mg/kg, n = 35). The different doses of mivacurium were injected before LMA insertion. The primary outcomes included the grading of conditions for the LMA insertion-18 score, onset time, recovery index and the duration that mivacurium was effective. Secondary outcomes included pulse oxygen saturation, mean blood pressure, heart rate and the incidence of adverse events.
Results: The score of the conditions for LMA insertion in Group A was significantly lower than in Groups C and B (p < 0.005). There was a significant difference in the onset time between Groups B and A (p < 0.005). There was no significant difference in the overall incidence of adverse reactions between these groups (p > 0.05).
Conclusion: Anaesthesia with 0.2 mg/kg of mivacurium can effectively shorten the onset time and facilitate insertion of the LMA in children undergoing day-case urologic surgery.
Keywords: anesthesiology; day-case surgery; laryngeal mask airway; mivacurium; urologic surgery in children.
© 2024 Ye, Nian, Zhou, Xie, Li, Xue and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The use of low-dose mivacurium to facilitate insertion of the laryngeal mask airway.Anaesthesia. 1998 May;53(5):491-5. doi: 10.1046/j.1365-2044.1998.00321.x. Anaesthesia. 1998. PMID: 9659025 Clinical Trial.
-
Randomised double-blind comparison of fentanyl, mivacurium or placebo to facilitate laryngeal mask airway insertion.Anaesthesia. 2000 Apr;55(4):323-6. doi: 10.1046/j.1365-2044.2000.01214.x. Anaesthesia. 2000. PMID: 10781116 Clinical Trial.
-
Comparison of the laryngeal mask (LMA) and laryngeal tube (LT) with the perilaryngeal airway (cobraPLA) in brief paediatric surgical procedures.Anaesth Intensive Care. 2008 May;36(3):425-30. doi: 10.1177/0310057X0803600314. Anaesth Intensive Care. 2008. PMID: 18564805 Clinical Trial.
-
Comparison of the laryngeal mask (LMA) and laryngeal tube (LT) with the new perilaryngeal airway (CobraPLA) in short surgical procedures.Eur J Anaesthesiol. 2006 Mar;23(3):234-8. doi: 10.1017/S0265021505002243. Eur J Anaesthesiol. 2006. PMID: 16430795 Clinical Trial.
-
[Effect of compound chamomile and lidocaine hydrochloride gel on oropharyngeal complications after the use of laryngeal mask airway with positive pressure ventilation].Zhonghua Yi Xue Za Zhi. 2023 Aug 22;103(31):2420-2426. doi: 10.3760/cma.j.cn112137-20230504-00720. Zhonghua Yi Xue Za Zhi. 2023. PMID: 37599216 Chinese.
References
LinkOut - more resources
Full Text Sources

