Classic and Atypical Late Infantile Neuronal Ceroid Lipofuscinosis in Latin America: Clinical and Genetic Aspects, and Treatment Outcome with Cerliponase Alfa
- PMID: 38469103
- PMCID: PMC10926189
- DOI: 10.1016/j.ymgmr.2024.101060
Classic and Atypical Late Infantile Neuronal Ceroid Lipofuscinosis in Latin America: Clinical and Genetic Aspects, and Treatment Outcome with Cerliponase Alfa
Erratum in
-
Corrigendum to "Classic and atypical late infantile neuronal ceroid lipofuscinosis in Latin America: Clinical and genetic aspects, and treatment outcome with cerliponase alfa." [Molecular Genetics and Metabolism ReportsVolume 38 (2024) 101060].Mol Genet Metab Rep. 2024 Apr 9;41:101081. doi: 10.1016/j.ymgmr.2024.101081. eCollection 2024 Dec. Mol Genet Metab Rep. 2024. PMID: 40206414 Free PMC article.
Abstract
Introduction: Late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), is a neurodegenerative autosomal recessive disease caused by TPP1 gene variants, with a spectrum of classic and atypical phenotypes. The aim of treatment is to slow functional decline as early as possible in an attempt to improve quality of life and survival. This study describes the clinical characteristics as well as the response to treatment with cerliponase alfa.
Materials and methods: A retrospective study was conducted in five Latin-American countries, using clinical records from patients with CLN2. Clinical follow-up and treatment variables are described. A descriptive and bivariate statistical analysis was performed.
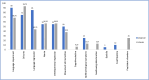
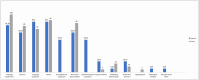
Results: A total of 36 patients were observed (range of follow-up of 61-110 weeks post-treatment). At presentation, patients with the classic phenotype (n = 16) exhibited regression in language (90%), while seizures were the predominant symptom (87%) in patients with the atypical phenotype (n = 20). Median age of symptom onset and time to first specialized consultation was 3 (classical) and 7 (atypical) years, while the median time interval between onset of symptoms and treatment initiation was 4 years (classical) and 7.5 (atypical). The most frequent variant was c.827 A > T in 17/72 alleles, followed by c.622C > T in 6/72 alleles. All patients were treated with cerliponase alfa, and either remained functionally stable or had a loss of 1 point on the CLN2 scale, or up to 2 points on the Wells Cornel and Hamburg scales, when compared to pretreatment values.
Discussion and conclusion: This study reports the largest number of patients with CLN2 currently on treatment with cerliponase alfa in the world. Data show a higher frequency of patients with atypical phenotypes and a high allelic proportion of intron variants in our region. There was evidence of long intervals until first specialized consultation, diagnosis, and enzyme replacement therapy. Follow-up after the initiation of cerliponase alfa showed slower progression or stabilization of the disease, associated with adequate clinical outcomes and stable functional scores. These improvements were consistent in both clinical phenotypes.
Keywords: Ataxia; Batten disease; CLN2; Cerliponase alfa; Enzyme replacement therapy; Epilepsy; Language delay; Pediatric.
© 2024 The Authors.
Conflict of interest statement
Dr. Atanacio has been a lecturer for the Biomarin Laboratory. Dr. Denzler has been a speaker for BioMarin. Dr. Durand has maintained a financial relationship with BioMarin through service contracts as speaker. Dr. Erlane has received travel grants, speaker fees, and educational grants from Biomarin. Dr. Espitia Segura reports a relationship with BioMarin Pharmaceutical Inc., including speaker fees and travel reimbursement. Dr. N Guelbert has received speaker fees from Biomarin. Dr. Pessoa has been a speaker for Biomarin. Dr. Naranjo and Dr. Tavera report a relationship with BioMarin Pharmaceutical Inc. that includes speaker and conference fees, as well as travel reimbursement.
Figures
Similar articles
-
Cerliponase Alfa for the Treatment of Atypical Phenotypes of CLN2 Disease: A Retrospective Case Series.J Child Neurol. 2021 May;36(6):468-474. doi: 10.1177/0883073820977997. Epub 2020 Dec 23. J Child Neurol. 2021. PMID: 33356800 Free PMC article.
-
"Real world effectiveness of cerliponase alfa in classical and atypical patients. A case series".Mol Genet Metab Rep. 2021 Feb 3;27:100718. doi: 10.1016/j.ymgmr.2021.100718. eCollection 2021 Jun. Mol Genet Metab Rep. 2021. PMID: 33604240 Free PMC article.
-
Presymptomatic treatment of classic late-infantile neuronal ceroid lipofuscinosis with cerliponase alfa.Orphanet J Rare Dis. 2021 May 14;16(1):221. doi: 10.1186/s13023-021-01858-6. Orphanet J Rare Dis. 2021. PMID: 33990214 Free PMC article.
-
Review of Cerliponase Alfa: Recombinant Human Enzyme Replacement Therapy for Late-Infantile Neuronal Ceroid Lipofuscinosis Type 2.J Child Neurol. 2020 Apr;35(5):348-353. doi: 10.1177/0883073819895694. Epub 2019 Dec 29. J Child Neurol. 2020. PMID: 31884868 Review.
-
Changing Times for CLN2 Disease: The Era of Enzyme Replacement Therapy.Ther Clin Risk Manag. 2020 Mar 30;16:213-222. doi: 10.2147/TCRM.S241048. eCollection 2020. Ther Clin Risk Manag. 2020. PMID: 32280231 Free PMC article. Review.
Cited by
-
Speech, Language and Non-verbal Communication in CLN2 and CLN3 Batten Disease.J Inherit Metab Dis. 2025 Jan;48(1):e12838. doi: 10.1002/jimd.12838. J Inherit Metab Dis. 2025. PMID: 39821609 Free PMC article.
References
-
- Gardner E., Bailey M., Schulz A., Aristorena M., Miller N., Mole S.E. Mutation update: review of TPP1 gene variants associated with neuronal ceroid lipofuscinosis CLN2 disease. Hum. Mutat. 2019 Nov 1;40(11):1924–1938. https://pubmed.ncbi.nlm.nih.gov/31283065/ [cited 2022 May 10]. Available from: - PMC - PubMed
-
- Kohan R., Cismondi I.A., Dodelson Kremer R., Muller V.J., Guelbert N., Tapia Anzolini V., et al. An integrated strategy for the diagnosis of neuronal ceroid lipofuscinosis types 1 (CLN1) and 2 (CLN2) in eleven Latin American patients. Clin. Genet. 2009;76(4):372–382. doi: 10.1111/j.1399-0004.2009.01214.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical