Cognitive performance in aged rats is associated with differences in distinctive neuronal populations in the ventral tegmental area and altered synaptic plasticity in the hippocampus
- PMID: 38469164
- PMCID: PMC10926450
- DOI: 10.3389/fnagi.2024.1357347
Cognitive performance in aged rats is associated with differences in distinctive neuronal populations in the ventral tegmental area and altered synaptic plasticity in the hippocampus
Abstract
Introduction: Deterioration of cognitive functions is commonly associated with aging, although there is wide variation in the onset and manifestation. Albeit heterogeneity in age-related cognitive decline has been studied at the cellular and molecular level, there is poor evidence for electrophysiological correlates. The aim of the current study was to address the electrophysiological basis of heterogeneity of cognitive functions in cognitively Inferior and Superior old (19-20 months) rats in the ventral tegmental area (VTA) and the hippocampus, having Young (12 weeks) rats as a control. The midbrain VTA operates as a hub amidst affective and cognitive facets, processing sensory inputs related to motivated behaviours and hippocampal memory. Increasing evidence shows direct dopaminergic and non-dopaminergic input from the VTA to the hippocampus.
Methods: Aged Superior and Inferior male rats were selected from a cohort of 88 animals based on their performance in a spatial learning and memory task. Using in vivo single-cell recording in the VTA, we examined the electrical activity of different neuronal populations (putative dopaminergic, glutamatergic and GABAergic neurons). In the same animals, basal synaptic transmission and synaptic plasticity were examined in hippocampal slices.
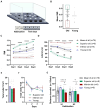
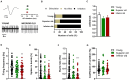
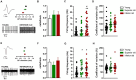
Results: Electrophysiological recordings from the VTA and hippocampus showed alterations associated with aging per se, together with differences specifically linked to the cognitive status of aged animals. In particular, the bursting activity of dopamine neurons was lower, while the firing frequency of glutamatergic neurons was higher in VTA of Inferior old rats. The response to high-frequency stimulation in hippocampal slices also discriminated between Superior and Inferior aged animals.
Discussion: This study provides new insight into electrophysiological information underlying compromised cerebral ageing. Further understanding of brain senescence, possibly related to neurocognitive decline, will help develop new strategies towards the preservation of a high quality of life.
Keywords: LTP; VTA GABAergic and VTA glutamatergic neurons; dementia; dopamine; in vivo electrophysiology; learning and memory.
Copyright © 2024 Sagheddu, Stojanovic, Kouhnavardi, Savchenko, Hussein, Pistis, Monje, Plasenzotti, Aufy, Studenik, Lubec and Lubec.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Persistent Adaptations in Afferents to Ventral Tegmental Dopamine Neurons after Opiate Withdrawal.J Neurosci. 2015 Jul 15;35(28):10290-303. doi: 10.1523/JNEUROSCI.0715-15.2015. J Neurosci. 2015. PMID: 26180204 Free PMC article.
-
Medial prefrontal cortical output neurons to the ventral tegmental area (VTA) and their responses to burst-patterned stimulation of the VTA: neuroanatomical and in vivo electrophysiological analyses.Synapse. 1999 Dec 15;34(4):245-55. doi: 10.1002/(SICI)1098-2396(19991215)34:4<245::AID-SYN1>3.0.CO;2-D. Synapse. 1999. PMID: 10529719
-
Spike timing-dependent plasticity at GABAergic synapses in the ventral tegmental area.J Physiol. 2013 Oct 1;591(19):4699-710. doi: 10.1113/jphysiol.2013.257873. Epub 2013 Jul 29. J Physiol. 2013. PMID: 23897235 Free PMC article.
-
NMDA receptors in the midbrain play a critical role in dopamine-mediated hippocampal synaptic potentiation caused by morphine.Addict Biol. 2014 May;19(3):380-91. doi: 10.1111/adb.12010. Epub 2012 Nov 19. Addict Biol. 2014. PMID: 23163242
-
Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine.Hippocampus. 2001;11(5):551-68. doi: 10.1002/hipo.1071. Hippocampus. 2001. PMID: 11732708 Review.
Cited by
-
A biophysical minimal model to investigate age-related changes in CA1 pyramidal cell electrical activity.PLoS One. 2024 Sep 4;19(9):e0308809. doi: 10.1371/journal.pone.0308809. eCollection 2024. PLoS One. 2024. PMID: 39231135 Free PMC article.
-
Dynamic changes in gene expression through aging in Drosophila melanogaster heads.G3 (Bethesda). 2025 Apr 17;15(4):jkaf039. doi: 10.1093/g3journal/jkaf039. G3 (Bethesda). 2025. PMID: 39992875 Free PMC article.
-
Dynamic Changes in Gene Expression Through Aging in Drosophila melanogaster Heads.bioRxiv [Preprint]. 2024 Dec 20:2024.12.11.627977. doi: 10.1101/2024.12.11.627977. bioRxiv. 2024. Update in: G3 (Bethesda). 2025 Apr 17;15(4):jkaf039. doi: 10.1093/g3journal/jkaf039. PMID: 39764034 Free PMC article. Updated. Preprint.
References
-
- Adrien J., Lanfumey L., Gozlan H., Fattaccini C. M., Hamon M. (1989). Biochemical and electrophysiological evidence for an agonist action of CM 57493 at pre-and postsynaptic 5-hydroxytryptamine1A receptors in brain. J. Pharmacol. Exp. Ther. 248, 1222–1230. PMID: - PubMed
LinkOut - more resources
Full Text Sources

