Conservation of shibire and RpII215 temperature-sensitive lethal mutations between Drosophila and Bactrocera tryoni
- PMID: 38469341
- PMCID: PMC10926519
- DOI: 10.3389/finsc.2024.1249103
Conservation of shibire and RpII215 temperature-sensitive lethal mutations between Drosophila and Bactrocera tryoni
Abstract
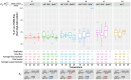
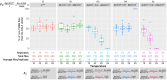
The sterile insect technique can suppress and eliminate population outbreaks of the Australian horticultural pest, Bactrocera tryoni, the Queensland fruit fly. Sterile males mate with wild females that produce inviable embryos, causing population suppression or elimination. Current sterile insect releases are mixed sex, as the efficient removal of unrequired factory-reared females is not yet possible. In this paper, we assessed the known Drosophila melanogaster temperature-sensitive embryonic lethal alleles shibire (G268D, shits1) and RNA polymerase II 215 (R977C, RpII215ts) for potential use in developing B. tryoni genetic sexing strains (GSS) for the conditional removal of females. Complementation tests in D. melanogaster wild-type or temperature-sensitive genetic backgrounds were performed using the GAL4-UAS transgene expression system. A B. tryoni wild-type shibire isoform partially rescued Drosophila temperature lethality at 29°C by improving survivorship to pupation, while expressing B. tryoni shits1 failed to rescue the lethality, supporting a temperature-sensitive phenotype. Expression of the B. tryoni RpII215 wild-type protein rescued the lethality of D. melanogaster RpII215ts flies at 29°C. Overexpressing the B. tryoni RpII215ts allele in the D. melanogaster wild-type background unexpectedly produced a dominant lethal phenotype at 29°C. The B. tryoni shibire and RpII215 wild-type alleles were able to compensate, to varying degrees, for the function of the D. melanogaster temperature-sensitive proteins, supporting functional conservation across species. Shibire and RpII215 hold potential for developing insect strains that can selectively kill using elevated temperatures; however, alleles with milder effects than shits1 will need to be considered.
Keywords: RNA polymerase II 215; embryo lethality; shibire; temperature sensitivity; transgenic complementation test.
Copyright © 2024 Nguyen, Choo and Baxter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors AC declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Precise single base substitution in the shibire gene by CRISPR/Cas9-mediated homology directed repair in Bactrocera tryoni.BMC Genet. 2020 Dec 18;21(Suppl 2):127. doi: 10.1186/s12863-020-00934-3. BMC Genet. 2020. PMID: 33339510 Free PMC article.
-
Lessons from Drosophila: Engineering Genetic Sexing Strains with Temperature-Sensitive Lethality for Sterile Insect Technique Applications.Insects. 2021 Mar 13;12(3):243. doi: 10.3390/insects12030243. Insects. 2021. PMID: 33805657 Free PMC article. Review.
-
Wild bacterial probiotics fed to larvae of mass-reared Queensland fruit fly [Bactrocera tryoni (Froggatt)] do not impact long-term survival, mate selection, or locomotor activity.Insect Sci. 2020 Aug;27(4):745-755. doi: 10.1111/1744-7917.12670. Epub 2019 Apr 21. Insect Sci. 2020. PMID: 30848568
-
Probable mechanisms underlying interallelic complementation and temperature-sensitivity of mutations at the shibire locus of Drosophila melanogaster.Genetics. 1998 Jun;149(2):1019-30. doi: 10.1093/genetics/149.2.1019. Genetics. 1998. PMID: 9611210 Free PMC article.
-
Parasitoids of Queensland Fruit Fly Bactrocera tryoni in Australia and Prospects for Improved Biological Control.Insects. 2012 Oct 22;3(4):1056-83. doi: 10.3390/insects3041056. Insects. 2012. PMID: 26466726 Free PMC article. Review.
References
-
- Kogan M, Bajwa W. Integrated pest management: A global reality? Anais da Sociedade Entomológica do Brasil. (1999) 28(1):1–25. doi: 10.1590/S0301-80591999000100001 - DOI
-
- Mau RFL, Jang EB, Vargas RI. The Hawaii area-wide fruit fly pest management programme: influence of partnerships and a good education programme. In: Area-Wide Control of Insect Pests. Springer Netherlands, Dordrecht: (2007).
-
- Vargas RI, Pinero JC, Leblanc L. An overview of pest species of Bactrocera fruit flies (Diptera: tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects. (2015) 6:297–318. doi: 10.3390/insects6020297 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

