Proteasome localization and activity in pig brain and in vivo small molecule screening for activators
- PMID: 38469354
- PMCID: PMC10925635
- DOI: 10.3389/fncel.2024.1353542
Proteasome localization and activity in pig brain and in vivo small molecule screening for activators
Abstract
Introduction: Loss of proteasome function, proteinopathy, and proteotoxicity may cause neurodegeneration across the human lifespan in several forms of brain injury and disease. Drugs that activate brain proteasomes in vivo could thus have a broad therapeutic impact in neurology.
Methods: Using pigs, a clinically relevant large animal with a functionally compartmental gyrencephalic cerebral cortex, we evaluated the localization and biochemical activity of brain proteasomes and tested the ability of small molecules to activate brain proteasomes.
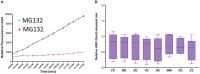
Results: By Western blotting, proteasome protein subunit PSMB5 and PSMA3 levels were similar in different pig brain regions. Immunohistochemistry for PSMB5 showed localization in the cytoplasm (diffuse and particulate) and nucleus (cytoplasm < nucleus). Some PSMB5 immunoreactivity was colocalized with mitochondrial (voltage-gated anion channel and cyclophilin D) and cell death (Aven) proteins in the neuronal soma and neuropil in the neocortex of pig and human brains. In the nucleus, PSMB5 immunoreactivity was diffuse, particulate, and clustered, including perinucleolar decorations. By fluorogenic assay, proteasome chymotrypsin-like activities (CTL) in crude tissue soluble fractions were generally similar within eight different pig brain regions. Proteasome CTL activity in the hippocampus was correlated with activity in nasal mucosa biopsies. In pilot analyses of subcellular fractions of pig cerebral cortex, proteasome CTL activity was highest in the cytosol and then ~50% lower in nuclear fractions; ~15-20% of total CTL activity was in pure mitochondrial fractions. With in-gel activity assay, 26S-singly and -doubly capped proteasomes were the dominant forms in the pig cerebral cortex. With a novel in situ histochemical activity assay, MG132-inhibitable proteasome CTL activity was localized to the neuropil, as a mosaic, and to cell bodies, nuclei, and centrosome-like perinuclear satellites. In piglets treated intravenously with pyrazolone derivative and chlorpromazine over 24 h, brain proteasome CTL activity was modestly increased.
Discussion: This study shows that the proteasome in the pig brain has relative regional uniformity, prominent nuclear and perinuclear presence with catalytic activity, a mitochondrial association with activity, 26S-single cap dominance, and indications from small molecule systemic administration of pyrazolone derivative and chlorpromazine that brain proteasome function appears safely activable.
Keywords: aging; chlorpromazine; encephalopathy; neonatal brain injury; proteasome nuclear satellite; protein aggregation; proteinopathy; pyrazolone.
Copyright © 2024 Amrein Almira, Chen, El Demerdash, Javdan, Park, Lee and Martin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
[Dynamic Changes in the Activities and Contents of Particular Proteasome Forms in the Cerebral Cortex of C57BL/6 Mice during Aging].Mol Biol (Mosk). 2023 Sep-Oct;57(5):886-894. Mol Biol (Mosk). 2023. PMID: 37752653 Russian.
-
Oleuropein Activates Neonatal Neocortical Proteasomes, but Proteasome Gene Targeting by AAV9 Is Variable in a Clinically Relevant Piglet Model of Brain Hypoxia-Ischemia and Hypothermia.Cells. 2021 Aug 18;10(8):2120. doi: 10.3390/cells10082120. Cells. 2021. PMID: 34440889 Free PMC article.
-
Polyclonal antibodies against human proteasome subunits PSMA3, PSMA5, and PSMB5.Hybridoma (Larchmt). 2012 Aug;31(4):272-8. doi: 10.1089/hyb.2012.0004. Hybridoma (Larchmt). 2012. PMID: 22894781
-
Proteasome Interactome and Its Role in the Mechanisms of Brain Plasticity.Biochemistry (Mosc). 2023 Mar;88(3):319-336. doi: 10.1134/S0006297923030033. Biochemistry (Mosc). 2023. PMID: 37076280 Review.
-
Regulation of proteasome complexes by gamma-interferon and phosphorylation.Biochimie. 2001 Mar-Apr;83(3-4):363-6. doi: 10.1016/s0300-9084(01)01249-4. Biochimie. 2001. PMID: 11295498 Review.
Cited by
-
Hypothermia Shifts Neurodegeneration Phenotype in Neonatal Human Hypoxic-Ischemic Encephalopathy but Not in Related Piglet Models: Possible Relationship to Toxic Conformer and Intrinsically Disordered Prion-like Protein Accumulation.Cells. 2025 Apr 12;14(8):586. doi: 10.3390/cells14080586. Cells. 2025. PMID: 40277911 Free PMC article.
References
-
- Adori C., Kovács G. G., Low P., Molnár K., Gorbea C., Fellinger E., et al. . (2005). The ubiquitin-proteasome system in Creutzfeldt-Jakob and Alzheimer disease: intracellular redistribution of components correlates with neuronal vulnerability. Neurobiol. Dis. 19, 427–435. doi: 10.1016/j.nbd.2005.01.015, PMID: - DOI - PubMed
-
- Almontashiri N. A., Chen H. H., Mailloux R. J., Tatsuta T., Teng A. C., Mahmoud A. B., et al. . (2014). SPG7 variant escapes phosphorylation-regulated processing by AFG3L2, elevates mitochondrial ROS, and is associated with multiple clinical phenotypes. Cell Rep. 7, 834–847. doi: 10.1016/j.celrep.2014.03.051, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

