SFX-01 in hospitalised patients with community-acquired pneumonia during the COVID-19 pandemic: a double-blind, randomised, placebo-controlled trial
- PMID: 38469377
- PMCID: PMC10926007
- DOI: 10.1183/23120541.00917-2023
SFX-01 in hospitalised patients with community-acquired pneumonia during the COVID-19 pandemic: a double-blind, randomised, placebo-controlled trial
Abstract
Introduction: Sulforaphane can induce the transcription factor, Nrf2, promoting antioxidant and anti-inflammatory responses. In this study, hospitalised patients with community-acquired pneumonia (CAP) were treated with stabilised synthetic sulforaphane (SFX-01) to evaluate impact on clinical status and inflammation.
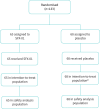
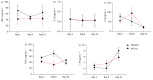
Methods: Double-blind, randomised, placebo-controlled trial of SFX-01 (300 mg oral capsule, once daily for 14 days) conducted in Dundee, UK, between November 2020 and May 2021. Patients had radiologically confirmed CAP and CURB-65 (confusion, urea >7 mmol·L-1, respiratory rate ≥30 breaths·min-1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years) score ≥1. The primary outcome was the seven-point World Health Organization clinical status scale at day 15. Secondary outcomes included time to clinical improvement, length of stay and mortality. Effects on Nrf2 activity and inflammation were evaluated on days 1, 8 and 15 by measurement of 45 serum cytokines and mRNA sequencing of peripheral blood leukocytes.
Results: The trial was terminated prematurely due to futility with 133 patients enrolled. 65 patients were randomised to SFX-01 treatment and 68 patients to placebo. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was the cause of CAP in 103 (77%) cases. SFX-01 treatment did not improve clinical status at day 15 (adjusted OR 0.87, 95% CI 0.41-1.83; p=0.71), time to clinical improvement (adjusted hazard ratio (aHR) 1.02, 95% CI 0.70-1.49), length of stay (aHR 0.84, 95% CI 0.56-1.26) or 28-day mortality (aHR 1.45, 95% CI 0.67-3.16). The expression of Nrf2 targets and pro-inflammatory genes, including interleukin (IL)-6, IL-1β and tumour necrosis factor-α, was not significantly changed by SFX-01 treatment. At days 8 and 15, respectively, 310 and 42 significant differentially expressed genes were identified between groups (false discovery rate adjusted p<0.05, log2FC >1).
Conclusion: SFX-01 treatment did not improve clinical status or modulate key Nrf2 targets in patients with CAP primarily due to SARS-CoV-2 infection.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: H.R. Keir reports receiving personal fees for educational lecture from Insmed Inc., outside the submitted work. Conflict of interest: A.T. Dinkova-Kostova participates on the Evgen Pharma Scientific Advisory Board, outside the submitted work. Conflict of interest: J.D. Chalmers reports support for the present manuscript from Lifearc; grants or contracts from AstraZeneca, Genentech, Gilead Sciences, GlaxoSmithKline, Insmed, Grifols, Novartis and Boehringer Ingelheim, outside the submitted work; consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, Insmed, Grifols, Novartis, Boehringer Ingelheim, Pfizer, Janssen, Antabio and Zambon, outside the submitted work; and is an associate editor of this journal. Conflict of interest: The remaining authors have nothing to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
