Hypoxia-activated ADCC-enhanced humanized anti-CD147 antibody for liver cancer imaging and targeted therapy with improved selectivity
- PMID: 38469549
- PMCID: PMC10927247
- DOI: 10.1002/mco2.512
Hypoxia-activated ADCC-enhanced humanized anti-CD147 antibody for liver cancer imaging and targeted therapy with improved selectivity
Abstract
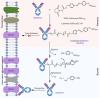
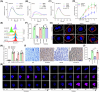
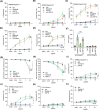
Therapeutic antibodies (Abs) improve the clinical outcome of cancer patients. However, on-target off-tumor toxicity limits Ab-based therapeutics. Cluster of differentiation 147 (CD147) is a tumor-associated membrane antigen overexpressed in cancer cells. Ab-based drugs targeting CD147 have achieved inadequate clinical benefits for liver cancer due to side effects. Here, by using glycoengineering and hypoxia-activation strategies, we developed a conditional Ab-dependent cellular cytotoxicity (ADCC)-enhanced humanized anti-CD147 Ab, HcHAb18-azo-PEG5000 (HAP18). Afucosylated ADCC-enhanced HcHAb18 Ab was produced by a fed-batch cell culture system. Azobenzene (Azo)-linked PEG5000 conjugation endowed HAP18 Ab with features of hypoxia-responsive delivery and selective targeting. HAP18 Ab potently inhibits the migration, invasion, and matrix metalloproteinase secretion, triggers the cytotoxicity and apoptosis of cancer cells, and induces ADCC, complement-dependent cytotoxicity, and Ab-dependent cellular phagocytosis under hypoxia. In xenograft mouse models, HAP18 Ab selectively targets hypoxic liver cancer tissues but not normal organs or tissues, and has potent tumor-inhibiting effects. HAP18 Ab caused negligible side effects and exhibited superior pharmacokinetics compared to those of parent HcHAb18 Ab. The hypoxia-activated ADCC-enhanced humanized HAP18 Ab safely confers therapeutic efficacy against liver cancer with improved selectivity. This study highlights that hypoxia activation is a promising strategy for improving the tumor targeting potential of anti-CD147 Ab drugs.
Keywords: cluster of differentiation 147; hypoxia activation; liver cancer; on‐target off‐tumor toxicity; therapeutic antibody.
© 2024 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
